| Pages:
1
2 |
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Growing Crystals in Gels
I found this really neat demonstration kit from Flinn - Growing Crystals in Gels - Chemical Demonstration Kit. In case the link ever gets broken, here's the text from that page:
| Quote: | Grow your own spectacular display of long-lasting crystals in gels—using large-size demonstration bottles! Mix two solutions together to form a gel
overnight. Add another solution to the hardened gel and watch amazing crystals begin to form before your eyes! Crystals become more and more beautiful
over the next two or three weeks! Introduce your students to the phenomenon of crystal growing with this easy-to-prepare demonstration. Highly visible
potassium hydrogen tartrate and lead iodide crystals are grown—making a perfect classroom display. Kit includes a detailed handout with
easy-to-follow instructions.
Concepts: Crystal formation, solutions/solubility, ions.
Setup Time Required: 30 minutes total over two class periods
Materials Provided: Acetic acid solution, lead nitrate solution, potassium chloride solution, sodium silicate solution, tartaric acid solution,
potassium iodide, and two crystal-growing bottles with caps. |
Very cool idea, but it's rather expensive. Based on the materials list, I would surmise the procedure to be something like this:
1) Mix acetic acid and sodium silicate solutions together, and add either lead nitrate or tartaric acid solution.
2) Allow to set up overnight.
3) Add either potassium iodide solution or potassium chloride solution (for lead nitrate and tartaric acid, respectively) on top of the gel, and the
crystals grow as the solution seeps down into it.
The problem then is to figure out amounts and concentrations. This would be something very cool to try if you've got the reagents and want to
experiment!
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
It sounds like a more controlled version of the chemical garden demonstration where various salts are dropped into a solution of sodium silicate.
I have not experimented at all with this. But I was at one stage considering electrolytically producing some tin crystals in a gel with the aim of
having them more durable than they would otherwise be.
I would be interested in anyone who could give precise recipes for growing crystals in gels.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4333
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I have a book on the subject in my office- I'll get back to you. And it's not like the chemical garden. The silicates are neutralized to form the
gel, so you get slow diffusion of cation and anion together to form the precipitate.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Quote: Originally posted by DraconicAcid  | | I have a book on the subject in my office- I'll get back to you. And it's not like the chemical garden. The silicates are neutralized to form the
gel, so you get slow diffusion of cation and anion together to form the precipitate. |
I stand corrected.
Looking forward to some wisdom from that book. 
|
|
|
pantone159
National Hazard
   
Posts: 590
Registered: 27-6-2006
Location: Austin, TX, USA
Member Is Offline
Mood: desperate for shade
|
|
The following is an excerpt from Chemical Demonstrations, A Handbook for Teachers of Chemistry, Bassam Z. Shakhashiri, Vol 3, 9.47, p372-377.
The source also has demonstrations including mercury crystals, which I did not copy here.
Sodium silicate solution, density c. 1.06 g/mL:
To prepare about 1 L of solution, dilute 160 mL of commercial 38-40% sodium silicate solution to 1.0 L with distilled water and stir the mixture.
Alternatively, dissolve 244 g of Na2SiO3-9H2O in 600 mL of distilled water, then dilute the resulting solution to 1 L.
Procedure A:
Preparation: Pour 15 mL of 1M acetic acid into a 50-mL beaker. Wearing gloves, add 2 mL of 1M lead acetate and stir the mixture.
While stirring the mixture vigorously, slowly pour 15 mL of the sodium silicate solution into the beaker. The mixture must be stirred continuously
while the silicate is added, because if any region of the mixture becomes basic, the lead will precipitate. Pour the mixture into a test tube,
and place in a rack until a gel forms (5-30 minutes).
Presentation: Pour 2 mL of 2M KI solution onto the top of the gel and stopper the tube. Place the tube where it can be observed undisturbed over
a period of several days. Compact yellow crystals of lead iodide will form, growing from the top of the gel toward the bottom.
Procedure B:
Preparation: Pour 15 mL of 1M acetic acid into a 50-mL beaker. Add 2 mL of 2M potassium iodide and stir the mixture.
While stirring the mixture vigorously, slowly pour 15 mL of the sodium silicate solution into the beaker.
Pour the mixture into a test tube, and place in a rack until a gel forms (5-30 minutes).
Presentation: Wearing gloves, pour 2 mL of 1M lead acetate solution onto the top of the gel and stopper the tube.
Place the tube where it can be observed undisturbed over a period of several days.
Yellow fern-like crystals of lead iodide will form in the gel, starting at the top and spreading toward the bottom.
Procedure E:
Preparation: Pour 15 mL of 1.5M tartaric acid into a 50-mL beaker.
While stirring the mixture vigorously, slowly pour 15 mL of the sodium silicate solution into the beaker.
Pour the mixture into a test tube, stopper the tube, and place in a rack until a gel forms (up to several days).
Presentation: Pour 2 mL of 1M CuSO4, 1M KCl, or 1M KNO3 solution onto the top of the gel and stopper the tube.
Place the tube where it can be observed undisturbed over a period of several days.
Crystals of either Cu tartrate or K tartrate will start at the top of the gel and grow downward.
[Edited on 24-7-2017 by pantone159]
|
|
|
tsathoggua1
Hazard to Others
  
Posts: 335
Registered: 8-1-2017
Location: Beyond the pale
Member Is Offline
Mood: Phosphorescent
|
|
Mercury crystals? presumably its still a liquid in the gel, or a Hg salt, no?
I'd be interested to see pics if it really is Hg(0) stabilized in solid form in a gel matrix though.
And as for acidifying silicate solutions, its not a bad way to prepare silicon. Adding concentrated sulfuric acid to potassium silicate solution
(again concentrated, not sure just how concentrated it is, since its a commercial product bought a long time back) to precipitate very very fine
silica, then washing out the potassium sulfate, each time decanting through filter paper after adding much cold H2O, allowing to stand until it
settles (its very, very fine) so takes a fair while, and removing the potassium sulfate. Once this is done, using Mg to reduce the precipitated silica
in a magnesiothermic or better, aluminothermic reduction. Al is IMO better since then one can test for the presence of Al residues by treatment with
strong aqueous NaOH or KOH. Tsath' did this, and he does need to refine the procedure in order to catch any vaporized material, he lost much of it the
last time he tried, using Al and carbon crucibles. Need something to direct and catch the vapors. But he did end up with 3 little nuggets of what can
only be silicon 'metal', silvery, hard, very very light and does not respond to concentrated sodium hydroxide or caustic potash solution in the
slightest. The only other elements that could have been present are hydrogen, oxygen, aluminium, the carbon of the crucibles or sulfur if residual
acid or sulfate were present, as well as potassium, and it quite definitely is none of these. Ergo it must be silicon.
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Thanks for that, pantone159! I'll have to look into trying this when I get back from some work trips.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4333
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Mercury(II) iodide and mercury(II) oxychloride.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
pantone159
National Hazard
   
Posts: 590
Registered: 27-6-2006
Location: Austin, TX, USA
Member Is Offline
Mood: desperate for shade
|
|
Yes, these Hg(II) compounds, not Hg metal.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4333
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I just set up a gel with tatrate, and will add some copper(II) sulphate solution to it once it has set, in accordance with the prophesy. I'll let you
know how it works.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4333
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Hmmmm...I got some very pale crystals in a blue solution (the gel melted over the summer
ETA: The gel didn't melt, there was just some supernatant over top of the gel. Nice little blue crystals, a lot like copper(II) sulphate. Tough to
separate them from the gel, though.
[Edited on 24-8-2017 by DraconicAcid]
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4333
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by DraconicAcid  | Hmmmm...I got some very pale crystals in a blue solution (the gel melted over the summer
ETA: The gel didn't melt, there was just some supernatant over top of the gel. Nice little blue crystals, a lot like copper(II) sulphate. Tough to
separate them from the gel, though.
[Edited on 24-8-2017 by DraconicAcid] |
I did get them separated, and put them in the oven at a mere 40 oC to dry. Now they are *very* pale blue- presumably dehydrated. Hmmm. Merck
doesn't say anything about copper(II) tartrate forming a hydrate. CRC says it's a trihydrate, but doesn't give a dehydration temp.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Neat! Very interesting results. I don't have any tartaric acid, so I was looking into a way to make it from cream of tartar. This thread has a few
options: http://www.sciencemadness.org/talk/viewthread.php?tid=21987
|
|
|
DraconicAcid
International Hazard
    
Posts: 4333
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Apparently, tartaric acid is readily available: https://www.walmart.com/ip/Tartaric-Acid-1lb/190132556 I'm also going to try it with glycinate.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
One thing this reminds me of is a series of kits that used to be available; they were each a big glass vial which you'd set a gel in and a chemical
reaction would take place. One of them was a typical Liesegang ring pattern that developed; I think it was called Flying Saucer Invasion or something.
There were others that had starburst or aurora patterns. I think they were available from Edmund Scientific but I've been unable to track them down.
Here's a few of my earlier experiments with Liesegang rings:
https://www.sciencemadness.org/whisper/viewthread.php?tid=26...
This thread got me motivated to test something that I'd been wondering about, which is what happened if a metal-rich gel was subjected to
electrolysis. I made a strong gelatin and copper sulfate solution and let it set; then I cut out a cube and left it on a strip of steel as an anode
and a wire as a cathode. I used a wall wart for a power supply.
Start:

End:

I had some trouble with dehydration, but I was definitely able to get a copper aggregate from the gel, though it seemed to take a lot longer than the
control aggregate I grew in ungelled water of the same copper concentration, with the same power supply (days vs. hours for a deposit of comparable
size). Another thing that struck me was that the gel-grown aggregate appeared to have a less branched, more cauliflower-like shape than the free-grown
aggregate. I don't know if this is because of hindered diffusion, or pressure from the constrictive gel.
Left, Copper aggregate grown in ungelled solution. Right, microscopic closeup
 
Left, Copper aggregate embedded in a gelatin matrix. The ion gradient and an opaque band of solid speckles are also visible. Right, microscopic
closeup.
 
The gel also develops a distinct gradient of color, where iron cations from the steel anode have migrated into the matrix. More subtle, but also
interesting, is the collection of solid speckles in the border between the oxidizing/iron and reducing/copper regions of the gel. These didn't form at
all in the leftover gel in the fridge; it's possible that they're the result of dehydration, but their placement is suggestive of some sort of
boundary phenomenon.
A thin slice displaying the ionic gradient, as well as speckles:

Gel, chunked by section: oxidizing/iron, boundary/speckles, reducing/copper, left-right.

Speckles, microphotograph:

al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Quote: Originally posted by DraconicAcid  | Quote: Originally posted by DraconicAcid  | Hmmmm...I got some very pale crystals in a blue solution (the gel melted over the summer
ETA: The gel didn't melt, there was just some supernatant over top of the gel. Nice little blue crystals, a lot like copper(II) sulphate. Tough to
separate them from the gel, though.
[Edited on 24-8-2017 by DraconicAcid] |
I did get them separated, and put them in the oven at a mere 40 oC to dry. Now they are *very* pale blue- presumably dehydrated. Hmmm. Merck
doesn't say anything about copper(II) tartrate forming a hydrate. CRC says it's a trihydrate, but doesn't give a dehydration temp.
|
I've done this prep as well. Unfortunately, the same thing happened to my, they dehydrated. I also recall that their crystal structure was rather
prismatic (I don't know my crystal structure names well). Also, the prep I used (admittedly an AP Chem prep) stated the product was Copper(II)
Hydrogen Tartrate (H2CuTar2, which would make more sense considering how copper usually coordinates with the tartrate ion (see
wikipedia for Fehling's) but sounds a bit simplified)
|
|
|
DraconicAcid
International Hazard
    
Posts: 4333
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I tried setting up gels for with benzoic acid, aspirin, and oxalic acid, but I thik i may have messed up the pH, as they didn't gel properly.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
lab-equip
Harmless

Posts: 13
Registered: 8-10-2016
Member Is Offline
Mood: No Mood
|
|
Cool stuff!
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
Quote: Originally posted by mayko  | | One thing this reminds me of is a series of kits that used to be available; they were each a big glass vial which you'd set a gel in and a chemical
reaction would take place. One of them was a typical Liesegang ring pattern that developed; I think it was called Flying Saucer Invasion or something.
There were others that had starburst or aurora patterns. I think they were available from Edmund Scientific but I've been unable to track them down.
|
From what I remember, the Flying Saucer Invasion looked a lot like this one, between copper sulfate and potassium chromate in silica:
https://www.physionet.org/tutorials/epn/html/chp62exp1.htm
I wondered if the products I was thinking of had been pulled for hexavalent chromium toxicity, but it looks like it's included in this kit, (not
available to the public at large though):
https://www.flinnsci.com/growing-crystals-in-gels---advanced...
In the course of looking for this I found this book, which turned out to be a pretty interesting, with lots of nice pictures (generally a pretty quick
read, though I'm going to have to sit down and look at some of the modelling):
https://www.amazon.com/Crystals-Liesegang-Rings-Heinz-Henisc...
Ch. 1: History & Nature of the Gel Method
Attachment: History_and_nature_of_the_gel_method.pdf (7.6MB)
This file has been downloaded 706 times
One neat thing mentioned here is the growth of crystals from a solution of a complex, diffusing and decomplexing.
| Quote: |
In the procedures described thus far, the gel is used as the reaction medium, in which the desired material is chemically formed. In a variant of the
method (...) for the growth of cuprous chloride crystals, the material is first complexed by means of another reagent (hydrochloric acid), and then
allowed to diffuse into a gel free of 'active' reagents. Decomplexing sets in with increasing dilution and leads to the high supersaturations
necessary for crystal growth. Cuprous chloride is interesting as a possible laser modulator, in which modulation can be achieved by a transverse
electrooptic effect (...) the initial experiments described by Armington and co-workers (1967a, b) were carried out in 2 cm diameter test tubes
containing pH 5 hydrochloric acid gels, with 5 ml of supernatant solution of varying acidity, saturated with cuprous chloride. Tetrahedral crystals of
3 mm size grew within about three weeks.
|
Silver iodide crystal growth is also mentioned, from diffusion of the complex with potassium iodide.
| Quote: |
There is evidence that the crystals grown in gels can be consider-
ably purer than the reagents used would lead one to expect (...) However general purification rules have not yet been established and, perplexing as
it may seem, some enrichment effects are also known (...) Of the crystals doped with the above elements, only those containing Mn were found to be
photoluminescent (orange) and cathodoluminescent (red). |
Ch. 2: "Gel Structure and Properties"
Attachment: Gel_structure_and_properties.pdf (1.8MB)
This file has been downloaded 489 times

Ch. 3: "Growth Mechanisms"
| Quote: |
The softness of the gel and, presumably, the uniform nature of the forces which it exerts upon the growing crystal make it possible to overdope
specimens until they are metastable as a result of severe internal strain. This can be demonstrated (Dennis, 1967), for instance, by adding a nickel
salt to a calcium tartrate growth system. Nickel is accommodated in the crystals, which become green as a result but remain perfectly clear. When the
amount of nickel exceeds a certain level, the crystal 'explodes' on contact, audibly if not violently. Nickel contents of the order of 1 % produce
this effect |
  
There's also this mention of pressure and solid growth in gel:
| Quote: |
For the measurement of crystallization pressures, Khaimov-Markov (1958) devised a method of disarming simplicity, as shown by the insert in Fig.
3.2.1. Seed crystals of aluminium potassium alum were placed on the top of a gel, with one of the crystal faces in contact with the gel surface. The
gel itself was supersaturated with solute, and as the crystal grew it was lifted up, eventually to a height of several millimetres. By placing weights
on the crystal, the lift could be suppressed, as shown by the graph in Fig. 3.2.\{a). In this way, and by monitoring crystal size, the pressure (i.e.
the thrust per unit area) could be simply determined. This pressure, in turn, was found to be a simple function of the super- saturation (Fig.
3.2.l(b)).
|
Ch. 4: "Nucleation"
Attachment: Nucleation.pdf (4.5MB)
This file has been downloaded 645 times
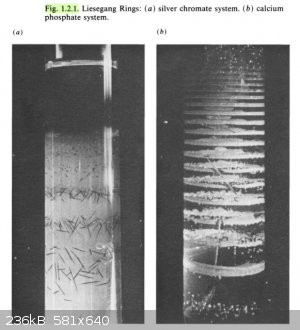
Ch. 5: "Liesegang Rings"
Attachment: Liesegang_Rings.pdf (7.2MB)
This file has been downloaded 842 times
Metallic mercury bands are apparently feasible; I wonder what they look like?
| Quote: | | Among the most exotic structures reported are (...) bands of metallic mercury, made by diffusing mercurous nitrate into an agar gel containing sodium
formate (Davies, 1917), |
There's also this mention of spiralling patterns; emergent spirals like this occur in other reaction-diffusion systems, like the B-Z reaction, though that might not be what's happening here.
| Quote: | | Liesegang (1939) subjected the Rothmund pictures to more detailed scrutiny, and found that they did not show circles at all, but concentric spirals
|
Also, light apparently has in impact upon development:
| Quote: | Hatschek (1921) discovered the light effect acciden- tally, and reported that the ring spacings in his lead chromate systems were different for
formation in darkness and under light. (...)Kiister (1913) worked on silver chromate deposition and obtained no
ring formation in his systems while these were kept in darkness, but prominent rings upon 'rhythmic' (i.e. intermittent, but otherwise unspecified)
illumination. Dhar and Chatterji (1934) did obtain rings in darkness, but found their number increased by light, and the spacing between them
diminished. Both Roy (1931) and Kisch (1933) reported that their rings were more distinct when formed 'under light' than in darkness.
|
/etc/
Attachment: Supplementary_notes_on_materials_grown_in_gels.pdf (672kB)
This file has been downloaded 522 times
Attachment: Measurements_on_gel-grown_crystals.pdf (263kB)
This file has been downloaded 496 times
Attachment: References.pdf (756kB)
This file has been downloaded 1047 times
The B. Vonnegut listed is Bernard, brother of Kurt 
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
DraconicAcid
International Hazard
    
Posts: 4333
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
So I'm getting little crystals of copper(II) benzoate, and the copper-oxalic acid mixture is giving interesting results. I believe I've got light
blue powdery bits of copper(II) oxalate, and needle-type crystals of darker blue sodium dioxalatocuprate.
[Edited on 28-9-2017 by DraconicAcid]

Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4333
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
My second try with copper acetate and aspirin in a gel is giving just a green solution, with no precipitate. Anthranilic acid is giving powdery
copper(II) anthranilate, so I may try that again with lower concentrations.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
I tried some copper-gelatin with oxalic acid solution but there just quickly formed a lot of powdery blue precipitate at the interface 
One cool thing about the copper-gelatin is how reactive it is towards other metals. It will easily chew a hole through aluminum foil, and seems to be
limited by dehydration as much as anything.

Here's what it did to a chunk of bulk aluminum:

This reaction creates dendritic copper metal better than any of the electrolysis attempts.
 
 
Even a single layer of aluminum foil will embed significant deposits in the gel matrix:
 
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Well, now you've got me curious. Would there be any benefits to reducing metals in a gel medium as opposed to an aqueous one? I'd imagine diffusion
would be much slower, allowing for more solid deposits (an explanation for why those 'trees' are so well-formed, maybe?), but I'm wondering if other
metals might be grown similarly, maybe even ones that ordinarily wouldn't grow well in water. I just bought some gelatin, so I'm about to try with
copper sulfate and an aluminum alloy plate.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
DraconicAcid
International Hazard
    
Posts: 4333
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I think the only benefit is that the mass of crystals is less likely to fall off the cathode.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
This is awesome work. Glad to see so much experimentation being done!
The copper dendrites in gel are particularly cool. So you made your copper gel (mixing gelatin into concentrated copper sulfate solution?) and just
laid it on top of aluminum foil? That's really interesting; I wonder how it eats through the oxide layer? Normally you need chloride ions for that.
Have you tried any other metal solutions? I'd really like to try this with things like nickel, lead, tin, etc. Those dendrites would make a cool
element sample.
|
|
|
| Pages:
1
2 |