Assured Fish
Hazard to Others
  
Posts: 319
Registered: 31-8-2015
Location: Noo Z Land
Member Is Offline
Mood: Misanthropic
|
|
Isoamyl bromide from isopropanyl bromide?
Is it possible to carry out an electrophilic addition reaction between isopropyl bromide and ethylene?
I figured that the carbon adjacent to the bromide is not electrophilic enough to attack the alkene but could it be accomplished with a catalyst, if so
what catalyst?
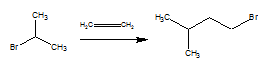
If this is a retarded question which will not work at all then feel free to throw it in detritus or delete it.
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
I doubt that route is feasible. Even if you could get the reaction to work, it would be hard to stop it after the addition of just one ethylene
molecule, so you would get a mixture of various oligomers.
|
|
|
Assured Fish
Hazard to Others
  
Posts: 319
Registered: 31-8-2015
Location: Noo Z Land
Member Is Offline
Mood: Misanthropic
|
|
Damn, this compound has annoyed me for quite some time, the only plausible easily accessible route to its synthesis that i can come up with is
reacting isobutyl magnesium bromide with anhydrous formaldehyde gas or some very dry paraformaldehyde in a grignard reaction. Isobutyl bromide could
be prepare from isobutyl nitrite which is sold as an OTC recreational inhalant in some countries.
|
|
|