NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Colourless gas from making copper nitrate
Just a curiosity question, well two actually.
I wanted some copper nitrate so added freshly stripped thick copper wire, to 50% Nitric Acid. To this I added ~100ml of cold water into the flask. I
was aware that it might take a while to react and for my purpose this was intended.
On top of the flask i placed a small round bottomed one parlty filled with cold water, the thinking being any Nitrogen dioxide would condense on the
bottom and drip back into the flask.
Fist day i got very few bubbles and a slight blue tinge to the flask, this was ok i was not in a hurry. Second day the water had reached room temp
(~16c) and a little more bubbling.
I had intended to move the flask that night but forgot....
In the morning I went to look and as i entered the room i could smell the nasty almost bleach like smell. Not bad enough to make you cough or anything
but enough to make me move it  . ANYWAY the flask has alot of tiny bubbles in
and still plenty of copper, it hasnt gone the deep blue i normally see. Instead its more a turquoise/green colour. . ANYWAY the flask has alot of tiny bubbles in
and still plenty of copper, it hasnt gone the deep blue i normally see. Instead its more a turquoise/green colour.
I assume this is just a hydration state as the acid was fairly strong and it hasnt finished reacting, but now the question.
The flask has small gaps around the round bottom one i put on top, i know there is a gas because i can see the tiny bubbles and i can smell it. As
soon as i lift off the small flask the neck of the main flask fills with Brown Nitrogen Dioxide.
If i place the flask back on then the gas slowly clears to colorless again.
The flask is slightly warm (~19c) room is around (~14c), I can see alot of tiny bubbles in the flask to the extent this might be making the colour
different, but what is the colourless gas?
Smells like nitrogen Dioxide just dosnt look at all brown until you lift the top flask off.
Sorry if its a stupid question but i am curious why the gas is clear until you lift the top. the neck isnt gas tight so i would assume air is getting
in anyway.
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Doh. its Nitric Oxide, lifting the flask on top allows more air in and produces Nitrogen Dioxide.
Found out by accident while searching for something else. i assume if i bubble this into water i get Nitrous acid?
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
well having moved it its gone from the blue colour a bit like copper hydroxide (could have been all the timy cuccles making the colour) to a really
deep clear emerald green colour!! the copper is slowly disolcing and a clear gas still evolving. any idea why the deep emerald green colour?? I was
expecting a deep blue colour???
|
|
|
DraconicAcid
International Hazard
    
Posts: 4334
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Online
Mood: Semi-victorious.
|
|
Quote: Originally posted by NEMO-Chemistry  | | well having moved it its gone from the blue colour a bit like copper hydroxide (could have been all the timy cuccles making the colour) to a really
deep clear emerald green colour!! the copper is slowly disolcing and a clear gas still evolving. any idea why the deep emerald green colour?? I was
expecting a deep blue colour??? |
It will be blue once you dilute it.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DraconicAcid  | Quote: Originally posted by NEMO-Chemistry  | | well having moved it its gone from the blue colour a bit like copper hydroxide (could have been all the timy cuccles making the colour) to a really
deep clear emerald green colour!! the copper is slowly disolcing and a clear gas still evolving. any idea why the deep emerald green colour?? I was
expecting a deep blue colour??? |
It will be blue once you dilute it. |
Really nice colour! Loads of copper left so i assume its used alot of the water up. I will transfer to a larger vessel and add some more water.
Thanks for the info, I have plenty Nitric acid and not a huge amount of conc sulphuric acid, I am actually after making copper sulphate, I can get it
locally but the price is a bit high and the quality is awful
|
|
|
CharlieA
National Hazard
   
Posts: 646
Registered: 11-8-2015
Location: Missouri, USA
Member Is Offline
Mood: No Mood
|
|
Just curious, but why did you expect nitrogen dioxide, mp ~ -11*C, to condense on your flask of cold water?
|
|
|
woelen
Super Administrator
        
Posts: 8014
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
The colorless gas is NO, which reacts with oxygen from air to form NO2. The following experiment is a nice demonstration of this reaction:
http://woelen.homescience.net/science/chem/exps/NO_O2/index....
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
I didnt exactly, i was a little lazy in my description. Normally when i do this i get brownish liquid droplets on the bottom of the water flask, I
assume this contains Nitrogen dioxide.
@Woelen
that is kind of exactly what I see in the flask, its a long necked flat bottomed round flask. The gas is clear but as soon as I remove the top flask
it goes brown. Pretty neat actually  . .
I also found if I stick a shovtwave UV lamp on top the flask the bubbles increase...but I guess everyone except me knew that 
|
|
|
ave369
Eastern European Lady of Mad Science
   
Posts: 596
Registered: 8-7-2015
Location: No Location
Member Is Offline
Mood: No Mood
|
|
The green color is, I think, some kind of complex. Copper 2+ goes green when it complexes with excess chloride ions, I guess it's the same with
nitrate ions.
Smells like ammonia....
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
dosnt help i took the pic on a blue top!! but i arowed the kind of green the flask has gone. loads of copper left so i assume its about out of water.
bubbles have stopped.
I am tempted to put some in a desiccator and see if i get any crystals and if so what colour etc
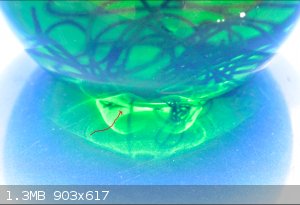
|
|
|
DraconicAcid
International Hazard
    
Posts: 4334
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Online
Mood: Semi-victorious.
|
|
Quote: Originally posted by NEMO-Chemistry  | dosnt help i took the pic on a blue top!! but i arowed the kind of green the flask has gone. loads of copper left so i assume its about out of water.
bubbles have stopped.
I am tempted to put some in a desiccator and see if i get any crystals and if so what colour etc |
If you get crystals, they'll be blue. But they tend to redissolve very easily.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
I was reading that they sublime in a vacuum at 180-200c (might have been thickypedia), I thought they would decompose fore reaching that temperature
though.
Totally ironic that the cheapest way for me to get half decent copper sulphate is with nitric acid!! Not sure how much longer my source of cheap acid
will last though.
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
ok something went wrong, i got tiny green sugar grain type crystals, the pic didnt come out well. As soon as you lift the desiccator they go blue and
mush, except a few that seem to stay green???
No real question just adding some observations as i loose notes  . .
|
|
|