Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
TLC with "shortwave" UV lamp and filter?
Quite a while back, I purchased some of these TLC plates, when the seller was just starting out and selling them for a lot less:
http://www.ebay.com/itm/182356708376
Of course, when I tested a mixture with it, and looked at it with a UV LED lamp, it looked nothing like the pictures, with only some dark spots being
faintly visible. But then I learned you're supposed to use "shortwave" UV for analyzing TLC plates, in order to do it properly. I also learned that
these waves should be below 300 or so nm, which requires a special lamp, and more importantly, a filter for that lamp, and they don't come cheap. So
do I need one of these lamps? Would it be really useful for me? If it's something I'd use a lot, then sure, I'd buy one. But would that be a
worthwhile investment?
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Plates like this are impregnated with a fluorophore, typically Mn-activated zinc silicate, which is fluorescent under 254 nm light (green). The eluted
compounds are revealed as dark spots because they are quenching the fluorescence (which limits the technique to quenching analytes, e.g. aromatics,
conjugated compounds, etc). Many lights, "mineralights", for example, will come complete with tubes that do 254 and 360-365nm, the latter being useful
for imaging fluorescent compounds on the plate (e.g. the spots). While less convenient than transilluminators, they are available at reasonable cost.
One of these, or the like, should do whatever you need: http://www.ebay.com/itm/ULTRA-VIOLET-UVP-MINERALIGHT-LAMP-MO...
O3
[Edited on 21-3-2017 by Ozone]
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
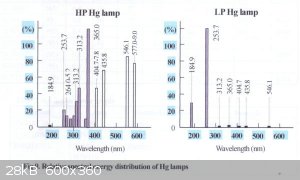
On the photo there's shown a perfect picture using an expensive 254 nm UV source that emmits no visible light at all. For basic applications you can
use a regular 254 nm low pressure mercury lamp, just make sure nothing is blocking the 254 nm light. Some bactericide lamps are made of a regular
glass that blocks 70% of light at 254 nm, thus it emmits much visible light compared to UV.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Thanks for that. My searches so far revealed mostly Chinese-made lamps for that price point, so I grabbed it up. The cheap LED lamp I was using
before didn't cast light evenly, so it was sometimes hard to tell what spots were actually a compound and what spots were just shadows. It was easier
to just use a developer. (iodine vapor then starch) But I figure there's enough use for a UVA/UVB lamp in chemistry to make this purchase worthwhile.
Do I need to wear eye protection when using this, or would it be sufficient to just point it away from me and never look at the light when it's on?
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Eye protection is recommended. Most safety glasses are ok, but you can get Uvex, if you like. Honestly, for casual use, just imaging plates, I've not
observed and symptoms whilst wearing standard, cheap PC safety glasses.
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
Texium
Administrator
       
Posts: 4581
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
I use a lamp just like that at work for looking at TLC plates and it works very well. I don't wear safety glasses when using it, but I point it away
from me and I do regularly wear prescription glasses which I'm sure are just as effective as cheap safety glasses, if not more, at blocking UV.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I've used those in organic classes. I don't remember wearing any special protective gear, but we were wearing goggles at the time and didn't stare
into the lamp. I do kind of prefer using a lamp to developing the plate.
|
|
|
Texium
Administrator
       
Posts: 4581
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Yeah, it's very convenient when you're always working with aromatic compounds that show up very well on the fluorescent plates and you have to run TLC
plates all the time.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
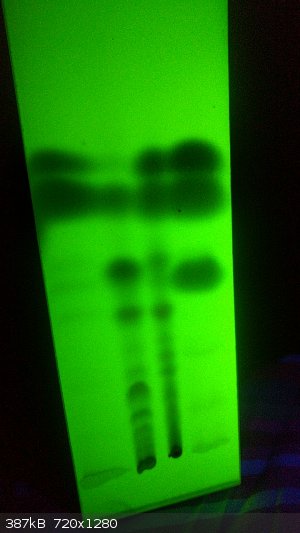
Ok, so I got the lamp in the mail, and apparently it's actually UVA and UVC. That's pretty cool. Each mode shows different patterns, both of which
were very helpful. I took two pictures of TLC plates, one under each type of light source. The short wave (green) is best for seeing contrast in
faint spots. The long-wave mode lights up each spot a different color. For a container, I used a tall 20 cm glass candleholder from a candle that I
bought in a Mexican dollar store. For the solvent, I didn't have any hexane and didn't feel like buying it mail-order. So I used Zippo lighter fluid
instead, (50/50 split with ethyl acetate) and it actually works extremely well.
|
|
|