A Halogenated Substance
Hazard to Self
 
Posts: 68
Registered: 7-2-2017
Location: United States
Member Is Offline
Mood: Oxidizing due to extended exposure to oxygen
|
|
Organic Polymer from Glycerol and Citric Acid Experiment
Background: A couple weeks ago, I was reading through an organic chemistry textbook and found that polymers could be made through
esterification of multi-alcohol and multi-carboxylic acid compounds.
The only multi-alcohol and multi-carboxylic acid I had on hand was glycerol and citric acid so I set off to confirm that they could be used to produce
a polymer.
And they can be!
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3972072/
I decided to do my own experiment with this process.
Process and Materials:
To start myself off, I wanted to try a 1:1 ratio (Important note: this was later changed to about 2:1) of glycerol to citric acid. To a 250 ml.
Erlenmeyer flask was added 40.0 grams of glycerol (0.436 mol) and 78.2 grams of citric acid (0.436 mol). However, the mixture was extremely thick and
gel-like. I had to try to mix it up several times as the citric acid in glycerol mixture made it feel like I was trying to stir a solid around. To fix
this, I added 44.7 grams of extra glycerol (0.488 mol) make the ratio of glycerol to citric acid about 2:1.
I then added about 3 ml. of concentrated sulfuric acid. It was unfortunately somehow contaminated in storage and was a brownish color but I didn't
consider this a major threat to the synthesis other than a color contamination (this can be seen later in photographs).
An interesting proportion of equal moles of glycerol and citric acid, side by side:

The apparatus as a whole:

The paper I used as a rough guide specified using reflux at temperatures around 90 and 150 degrees Celsius. I placed the mixture in reflux for 2 and a
half hours at around 100 degrees Celsius to try and follow that range.
After letting it cool, I washed the product with 100 milliliters of distilled water (which floated on top without aggravation with stirring). I set
the remaining mixture to boil at a little above 100 degrees Celsius on a hotplate (Note: the mixture was liquid and free flowing at this temperature
range.
The product was yet allowed to cool and it solidified into a very hard-to-manipulate substance that I expect to be the desired polymer. The mass of it
turned out to be 64.2 grams.
 
I additionally expect it to be extremely impure.
I'm not sure how I would calculate an exact 'yield' based on a polymer, especially considering the imperfect 2:1 ratio between glycerol citric acid.
Instead, I decided to base it upon the starting and final masses.
And with 163 grams of reactants and 64.2 grams of product, I came out with 39% of the original mass under the assumption that a perfect yield would
include the complete reaction between glycerol and citric acid.
Now it's time to find a solvent to help clean both my product and glassware. Yay. (I did notice however that acetone seemed to help clean and dissolve
the polymer a bit. I think I'll try chlorinated solvents next though)
Ideas or comments? Any would be appreciated! Thank you!
Current Active works in Progress:
-Convert i-propanol to i-chloropropane using HCl and ZnCl2
-Determine properties and solubility of a glycerol and citric acid polymer
-Attempt a conversion of chloroform to dichloromethane
-Attempt a conversion of acetic acid to acetamide
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Good Job but:
There is no need to do this reaction under reflux(beaker is better for synthesis this polyester)
Esterification will proceed if formed water come out and reflux apparatus prevent it.
Do not heat reaction more than 120 C otherwise citric acid decompose.
Use PTSA(P-toluenesulfonic Acid) instead of sulfuric acid(this will prevent unwanted products),1% by weigh is enough
Try Acetone or MIBK for solvent and DCM or Ether for cleaning
By using different molar ratio of glycerine and citric acid you can make Hyperbranched Polyester.
I have experience on synthesis of Polymers (specially Polyesters).
Hyperbranched poly(citric acid) and its application as anticancer drug delivery system
http://onlinelibrary.wiley.com/doi/10.1002/app.39028/abstract
[Edited on 19-3-2017 by Waffles SS]
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Interesting stuff, I wonder if it acts as a base exchange polymer? Is it hydrophilic ie wettable and does it swell in dilute alkali?
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Finding out how it behaves in the presence of alkali may solve two problems- it might answer Boffis' question, and it might (with luck) also address
the issue of a solvent to clean the glassware with.
|
|
|
A Halogenated Substance
Hazard to Self
 
Posts: 68
Registered: 7-2-2017
Location: United States
Member Is Offline
Mood: Oxidizing due to extended exposure to oxygen
|
|
Quote: Originally posted by Boffis  | | Interesting stuff, I wonder if it acts as a base exchange polymer? Is it hydrophilic ie wettable and does it swell in dilute alkali?
|
Before I boiled it down, any additionally water sat as a separate layer on top.
However, I'm away from the house for a week so I can't perform any additional tests on it for a while.
On another note, it did seem soluble in glycerol and when I used water to try rid of the glycerol and the citric acid impurities, it thickened quite a
bit until I had to use a stir rod to transport it into another container.
Current Active works in Progress:
-Convert i-propanol to i-chloropropane using HCl and ZnCl2
-Determine properties and solubility of a glycerol and citric acid polymer
-Attempt a conversion of chloroform to dichloromethane
-Attempt a conversion of acetic acid to acetamide
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Cool experiment. If you have to, you can surely clean the polymer with piranha solution, but I'd clean as much off as possible with, for example,
water, acetone, sodium hydroxide solution, or brake cleaner and scraping, etc. first. If you have abundant quantities of it or are extremely wealthy
or something, DCM usually works better than brake cleaner for cleaning glass, but in my book, it's pretty much a last resort before using piranha.
Also, I'd apply the piranha dropwise, outside, wearing full lab protective gear, in case there is a violent reaction.
|
|
|
Outer
Harmless

Posts: 38
Registered: 24-11-2008
Member Is Offline
Mood: No Mood
|
|
You can also try the same reaction in a microwave oven.
|
|
|
PirateDocBrown
National Hazard
   
Posts: 570
Registered: 27-11-2016
Location: Minnesota
Member Is Offline
Mood: No Mood
|
|
A good chunk of my professional career was in the development of just such condensation polymers.
A great deal of crosslinking would be done here, since you are using tribasic monomers.
Switching to dibasic ones, like glycols for the hydroxy monomer and like phthalates or other dicarboxylates (malonate or succinate) for the acid, will
give you much greater control of the outcomes.
If crosslinking is desired, dicarboxylates like fumarate or malate can be used, so crosslinking can be attained via free-radical chain reaction
(initiated by some peroxide). This would be facilitated by an admixture of further unsaturated monomer, such as styrene or cyclopentadiene (usually as
the dimer). Additionally, cyclopentadiene can be added to the original chains via Diels-Alder addition, extending a double bond away from the main
polyester chain.
There are literally thousands of recipes that have been developed over the decades.
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1640
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
Now this stuff I am very interested as I make allot of custom stuff and want to learn a shit ton more about plastic and rubber making to use in feed
throughs and the like for small custom vacuum systems.
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
Here's another interesting polymer using citric acid as a cross-linking agent:
Altuna, F. I., Pettarin, V., & Williams, R. J. J. (2013). Self-healable polymer networks based on the cross-linking of epoxidised soybean oil by
an aqueous citric acid solution. Green Chemistry, 15(12), 3360. http://doi.org/10.1039/c3gc41384e
| Quote: |
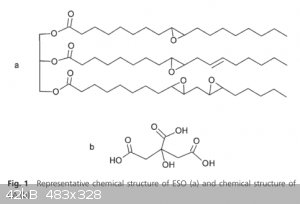
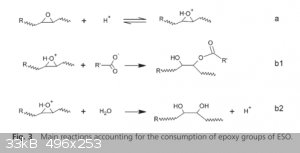
Epoxidised soybean oil (ESO) was cross-linked with an aqueous citric acid (CA) solution without the addition of any other catalyst or solvent.
Completely bio-based polymer networks were generated. The initial system was an emulsion, but it became a homogeneous and transparent polymer network
by reac- tion. The ability of the final materials to self-heal without adding extrinsic catalysts was assessed by stress relaxation and lap-shear
tests. This was achieved by molecular rearrangements produced by thermally activated transesterification reactions of β-hydroxyester groups generated
in the polymerization reaction.
|
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
Chemetix
Hazard to Others
  
Posts: 376
Registered: 23-9-2016
Location: Oztrayleeyah
Member Is Offline
Mood: Wavering between lucidity and madness
|
|
Sugar as either the mono or di saccharide with citric and an amine makes a dark polymer goop, but a BBQ will do exactly that. The amine from protein
and the citric acid from cyto-cellular sources and some blood sugar gives the characteristic brown colour to cooked meat.
I have to say that citric and sugar as a solution works as a topical antibacterial par excellence. I assume that the outer membrane of the bacteria
gets 'burnt' as the proteins gets polymerised with the sugar and citric. I think that the bacterial cells also get stressed, making the decision
between operating proton pumps and fighting the osmotic pressure of the sugar. A small vial sits on my bathroom sink as our 'liquid spot remover'.
There's a PhD in that for someone I'm sure.
|
|
|
j_sum1
Administrator
       
Posts: 6334
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Quote: Originally posted by Chemetix  | | The amine from protein and the citric acid from cyto-cellular sources and some blood sugar gives the characteristic brown colour to cooked meat.
|
Hm. I thought the brown came from the Maillard reaction and involved amines in proteins and aldehyde groups originating from sugars -- forming a
complex set of imines.
I will have to try that citric acid / sugar polymerisation some time though.
|
|
|
Chemetix
Hazard to Others
  
Posts: 376
Registered: 23-9-2016
Location: Oztrayleeyah
Member Is Offline
Mood: Wavering between lucidity and madness
|
|
leave a solution of sugar and citric on the bench, then add some urea to another solution of citric- sugar, the one with urea goes brown.
|
|
|
joeedh
Harmless

Posts: 2
Registered: 10-7-2017
Member Is Offline
Mood: No Mood
|
|
I've done this myself. It's a bit tricky to control; the longer you microwave it the more cross-linked it becomes. I got the idea from a paper I
found online somewhere.
|
|
|