| Pages:
1
2 |
LD5050
Hazard to Others
  
Posts: 182
Registered: 16-1-2017
Member Is Offline
Mood: No Mood
|
|
Can I concentrate my 68% nitric acid in to 90%+?
How did you get 90% nitric acid I thought the highest concentration was 68% How d you break the azeotrope?
[Edited on 2-22-2017 by zts16]
|
|
|
Cryolite.
Hazard to Others
  
Posts: 269
Registered: 28-6-2016
Location: CA
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by LD5050  | | How did you get 90% nitric acid I thought the highest concentration was 68% How d you break the azeotrope? |
You just distill nitric acid by mixing a nitrate salt and sulfuric acid. If there is only a small amount of water in the acid and the salt, the nitric
acid which comes off can only contain this little bit of water, and will therefore be stronger than azeotropic.
|
|
|
LD5050
Hazard to Others
  
Posts: 182
Registered: 16-1-2017
Member Is Offline
Mood: No Mood
|
|
Can I concentrate my 68% nitric acid in to 90%+?
I made some azeotropic nitric acid by distilling a mixture of sulfuric acid, ammonium nitrate, and water. I know have 68% Nitric acid but I need a
more concentrated form to oxidize Benzyl alcohol into Benzaldehyde. Is there any way to break the azeotropic nitric acid that I already have? I don't
believe I am able to distill fuming nitric acid from H2SO4 and ammonium nitrate because ammonia gas produced will neutralize the nitric acid.
|
|
|
LD5050
Hazard to Others
  
Posts: 182
Registered: 16-1-2017
Member Is Offline
Mood: No Mood
|
|
I only have ammonium nitrate tho and it produces ammonia gas and neutralizes the nitric acid so Is there any way to concentrate the 68 to 90%?
|
|
|
Lambda-Eyde
National Hazard
   
Posts: 860
Registered: 20-11-2008
Location: Norway
Member Is Offline
Mood: Cleaved
|
|
Yes, there is. You can distill it again with more concentrated sulfuric acid to retain the water. However, if you didn't know this or couldn't find
this out on your own I don't think it's a very good idea. Fuming nitric acid is seriously nasty stuff even when you're not distilling it. The
distillation process produces some nitrogen oxides as well.
This just in: 95,5 % of the world population lives outside the USA
Please drop by our IRC channel: #sciencemadness @ irc.efnet.org
|
|
|
Texium
Administrator
       
Posts: 4581
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by LD5050  | | I only have ammonium nitrate tho and it produces ammonia gas and neutralizes the nitric acid so Is there any way to concentrate the 68 to 90%?
|
It shouldn't, when you have sulfuric acid in there. You'll be left with ammonium sulfate, which doesn't
begin to decompose until well over 200ºC. Ammonium nitrate is fine, though potassium nitrate or calcium ammonium nitrate is preferable.
|
|
|
Texium
|
Threads Merged
21-2-2017 at 19:04 |
Texium
|
Thread Moved
21-2-2017 at 19:05 |
LD5050
Hazard to Others
  
Posts: 182
Registered: 16-1-2017
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Lambda-Eyde  | | Yes, there is. You can distill it again with more concentrated sulfuric acid to retain the water. However, if you didn't know this or couldn't find
this out on your own I don't think it's a very good idea. Fuming nitric acid is seriously nasty stuff even when you're not distilling it. The
distillation process produces some nitrogen oxides as well. |
I did a quick search on google and through other sources but couldn't find much other than starting from scratch which I didn't want to do, and also I
only had ammonium nitrate and read that it cant be done because it neutralizes the acid. I figured I would probably be able to distill the 68% from
more H2SO4 to obtain a higher concentration but i wasn't sure so I asked on here. Thanks tho, and I do know the dangers associated with concentrated
acids. What sulfuric to nitric acid molar ratio should I prepare in the boiling flask when doing this? I'm sure its not super important to get it
precise.
|
|
|
Texium
Administrator
       
Posts: 4581
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
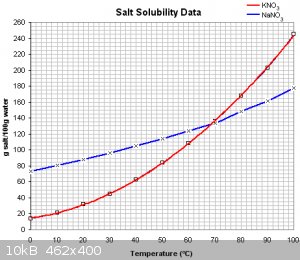
To be honest, you're overthinking it. Your best bet is to prepare sodium or potassium nitrate by adding your ammonium nitrate to a boiling solution of
NaOH or KOH (or carbonate if you don't have a hydroxide available). Ammonia gas will be driven off, and you'll be left with a solution of the pure
alkali nitrate. I'd recommend potassium over sodium because it is much easier to crystallize (if your boiling solution is fairly concentrated, you
should be able to recover most of it just by cooling it).
|
|
|
LD5050
Hazard to Others
  
Posts: 182
Registered: 16-1-2017
Member Is Offline
Mood: No Mood
|
|
Ya I actually just did this procedure, 200g ammonium nitrate and and 100g of sodium hydroxide. I didn't boil it tho except from it boiling on its own
for a couple minutes after I poured in the water. After the reaction died down I put some more water in (around 200ml total) and mixed it around and
then filtered which was a pain. I put it in the freezer to initiate crystalization but not much seemed to happen so I boiled off some of the water
until I saw some precipitate and now i got it cooling on the counter. I see it crystalizing out but the yield seems pretty low.
|
|
|
chornedsnorkack
National Hazard
   
Posts: 563
Registered: 16-2-2012
Member Is Offline
Mood: No Mood
|
|
Can you end up with above 100 % nitric acid?
|
|
|
LD5050
Hazard to Others
  
Posts: 182
Registered: 16-1-2017
Member Is Offline
Mood: No Mood
|
|
How would you come up with nitric acid above 100%?...thats impossible...
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Nitric acid with its dehydrate N2O5 dissolved in it is referred to as being more than 100%
|
|
|
Herr Haber
International Hazard
    
Posts: 1236
Registered: 29-1-2016
Member Is Offline
Mood: No Mood
|
|
STOP ADDING WATER !
Only add water to your round bottom flask AFTER the distillation to get rid of the sulphate salt.
Adding water when you are trying to get a concentrate is a bit counterproductive to say the least.
|
|
|
Fulmen
International Hazard
    
Posts: 1716
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Equal volumes of 98% H2SO4 and 65% HNO3 is a good place to start.
There is also a process using magnesium nitrate instead of sulfuric acid. Never tried it but it could be easier to regenerate the magnesium nitrate.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
chornedsnorkack
National Hazard
   
Posts: 563
Registered: 16-2-2012
Member Is Offline
Mood: No Mood
|
|
What´s better to concentrate nitric acid: H2SO4 or P2O5?
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
N2O5 is far better 
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Booze
Hazard to Others
  
Posts: 121
Registered: 26-2-2017
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by LD5050  | | I made some azeotropic nitric acid by distilling a mixture of sulfuric acid, ammonium nitrate, and water. I know have 68% Nitric acid but I need a
more concentrated form to oxidize Benzyl alcohol into Benzaldehyde. Is there any way to break the azeotropic nitric acid that I already have? I don't
believe I am able to distill fuming nitric acid from H2SO4 and ammonium nitrate because ammonia gas produced will neutralize the nitric acid.
|
When you did this, did you distill nitrogen dioxide? I have been trying to do this for some time. Any tips?
|
|
|
ficolas
Hazard to Others
  
Posts: 146
Registered: 14-5-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Booze  |
When you did this, did you distill nitrogen dioxide? I have been trying to do this for some time. Any tips? |
Do you, for some weird reason want nitrógen dioxide, or do you want nitric acid? As somebody alredy told you, nitrogen dioxide is a product of the
decomposition of nitrix acid, if you want nitric acid, you dont need to "distill nitrogen dioxide"
|
|
|
Fulmen
International Hazard
    
Posts: 1716
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Define "better".
P2O5 is a potent chemical, capable of dehydrating even stubborn chemicals/mixtures. But regenerating phosphoric acid back to P2O5 isn't possible. So
unless you have good access to P2O5 and have a use for the phosphoric acid H2SO4 will be better as it can be re-concentrated by distillation.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
chornedsnorkack
National Hazard
   
Posts: 563
Registered: 16-2-2012
Member Is Offline
Mood: No Mood
|
|
Just to remind: the pure compounds in the system are:
HNO3 - boils at +83, freezes at -42
N2O5 - melts at +41, boils at +47
N2O4/NO2 - boils at +22, freezes at -11.
|
|
|
LD5050
Hazard to Others
  
Posts: 182
Registered: 16-1-2017
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Booze  | Quote: Originally posted by LD5050  | | I made some azeotropic nitric acid by distilling a mixture of sulfuric acid, ammonium nitrate, and water. I know have 68% Nitric acid but I need a
more concentrated form to oxidize Benzyl alcohol into Benzaldehyde. Is there any way to break the azeotropic nitric acid that I already have? I don't
believe I am able to distill fuming nitric acid from H2SO4 and ammonium nitrate because ammonia gas produced will neutralize the nitric acid.
|
When you did this, did you distill nitrogen dioxide? I have been trying to do this for some time. Any tips? |
Very little brown/red nitrogen dioxide was formed I also used NileRed method. After the first distillation the acid will be pretty weak especially if
its cold. I added this to some copper with not much effect at all. I then proceeded with a fractional distillation and collected everything after 120c
and ended up with azeotropic nitric acid which I got a VERY strong reaction when added to copper. The entire process is pretty straight forward I'm
confused as to what you are having trouble with ( I saw your other post). I would suggest to fractionally distill the distillate after the first
distillation.
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
You don't need highly concentrated nitric acid to oxidize benzyl alcohol.
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
Tsjerk is correct; if you even read my original benzaldehyde thread you'd have seen all the papers that achieved even better results than mine using
dilute nitric acid and a small amount of nitrite.
|
|
|
chornedsnorkack
National Hazard
   
Posts: 563
Registered: 16-2-2012
Member Is Offline
Mood: No Mood
|
|
Azeotropic, 68 % nitric acid is near an eutectic at about 71 %.
|
|
|
LD5050
Hazard to Others
  
Posts: 182
Registered: 16-1-2017
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Amos  | | Tsjerk is correct; if you even read my original benzaldehyde thread you'd have seen all the papers that achieved even better results than mine using
dilute nitric acid and a small amount of nitrite. |
Amos I did in fact read your thread and I wanted to try the 10% nitric acid method but I didn't have any nitrite and without out it the yields aren't
to great.
|
|
|
| Pages:
1
2 |