D4RR3N
Hazard to Others
  
Posts: 271
Registered: 9-1-2007
Member Is Offline
Mood: No Mood
|
|
Celery contains androsterone
How would you go about extracting it and concentrating it ?
btw androsterone is supposed to be a pheromone 
|
|
|
A Halogenated Substance
Hazard to Self
 
Posts: 68
Registered: 7-2-2017
Location: United States
Member Is Offline
Mood: Oxidizing due to extended exposure to oxygen
|
|
Well, you could instead extract about 50 milligrams of it from 17,000 liters of male urine like it says on wikipedia.
https://en.wikipedia.org/wiki/Androsterone
Why do you need a weak derivative of testosterone? The  is quite suggestive. is quite suggestive.
[Edited on 2-12-2017 by A Halogenated Substance]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
First you need a computer and an internet connection - so far so good.
Next there is this website called 'google' that has answers to many questions, also pointers to other places to look.
After that, there are strange places called 'libraries' that contain things called 'books' (a kind of primitive web page printed on bits of paper).
Some wierdos used to try to do it themselves first without being spoon-fed !
Odd, possibly illegal these days, but true.
|
|
|
Texium
|
Thread Moved
12-2-2017 at 12:15 |
D4RR3N
Hazard to Others
  
Posts: 271
Registered: 9-1-2007
Member Is Offline
Mood: No Mood
|
|
Celery is more fun then to play with gallons of piss I guess 
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
You can probably buy aerosol cans of it at your farm supply shop for just 7 euros/£/$. It works on sows at the right time in their cycle. If your
planning some experiments with other species do post the results. At least you can guantee you will get lucky with a sow eventually LOL

PS sorry for the huge pic.
[Edited on 12-2-2017 by wg48]
|
|
|
Assured Fish
Hazard to Others
  
Posts: 319
Registered: 31-8-2015
Location: Noo Z Land
Member Is Offline
Mood: Misanthropic
|
|
Pancuronium bromide from Androsterone maybe?
A disscusion regarding this might be better suited for the organics section but since this thread has already began i will start here.
Pancuronium bromide
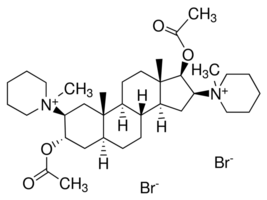
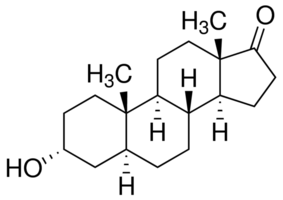
Androsterone
Preparation of a similar compound from a similar starting material

Ok first things first we would need to dehydrate the Androsterone to form the desired cycloalkene (I do not know how to name molecules of this size it
would be nice if someone could point me in the direction of somewhere i could learn), This would have to be done in liquid phase however the only ways
i know how to dehydrate an alcohol are by dehydration over aluminium oxide or acid catalyzed dehydration both of which require rather high
temperatures and i doubt the androsterone would survive these conditions, so perhaps someone would have a better idea about how we could do that?
The next step would be acetylation of the ketone using mercury sulfate (dangerous but doable) and an unfamiliar acetylating agent which yields acetone
as a co-product (my nomenclature knowledge hit a barrier here so excuse if i am incorrect but i think its 1-methyl ethene ethanoate).
However i see no reason why this couldn't be done with acetic anhydride with the co-product being acetic acid instead.
The next step is an epoxidation using meta-chloroperoxybenzoic acid or mCPBA, which may be difficult to prepare because a benzoyl chloride must be
prepared which requires a powerful chlorinating agent although i do recall magpie or somebody preparing a benzoyl chloride from benzoic acid using HCl
but i could be wrong on this. Non the less the resulting epoxide would then have to be reacted with piperidine to form the next step.
https://en.wikipedia.org/wiki/Meta-Chloroperoxybenzoic_acid
The next step is straight forward as hell and is just a hydrogenation to reduce the ketone to an alcohol (this could be acheived with Pd/C & H2,
NaBH4, LAH, or maybe even AlHg respectively.
The last steps again are rather straight forward and involve a acetylation of the alcohols using acetic anhydride and finally a reaction with
bromomethane in a Menshutkin/quarternization reaction to finally yield what is now im guessing a water soluble product.
I see no reason why an amateur could not use iodomethane in place of bromomethane to yield a still active product.
Cheers Fish
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Tough decision. I have 17,000 Liters of my own urine, stored in old pop-bottles, down in my catacombs, but I have been saving it for an Alchemy
experiment.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
how is the regioselectivity of this reaction controlled,especially for the epoxide on the left ?
|
|
|
Corrosive Joeseph
National Hazard
   
Posts: 915
Registered: 17-5-2015
Location: The Other Place
Member Is Offline
Mood: Cyclic
|
|
Can I just clarify....................
AndrosteRone............. https://en.wikipedia.org/wiki/Androsterone is in piss...............
AndrosteNone........... https://en.wikipedia.org/wiki/Androstenone is in boar saliva, celery and truffle fungus..............
OP is wrong, me thinks...................... Maybe caused by a state of over-excitement.................
And while l'm at it, this was posted in the wrong forum........................ But has since been moved.................... TF
/CJ
Being well adjusted to a sick society is no measure of one's mental health
|
|
|
Assured Fish
Hazard to Others
  
Posts: 319
Registered: 31-8-2015
Location: Noo Z Land
Member Is Offline
Mood: Misanthropic
|
|
| Quote: |
how is the regioselectivity of this reaction controlled,especially for the epoxide on the left ?
|
Good question, to be honest i never even thought about the sterioisomers and which ones would be active or inactive however i googled it and found
this paper, which describes the reaction of amine (specifically aniline) with an epoxide using a catalyst under solvent free conditions to achieve a
sterioselective product, I don't know which isomer would be favorable if we were to carry out this reaction on the compound in question but its at
least a step in the right direction.
It describes using mostly a catalyst called SBSSA but in a table it shows that they carried out the reaction using slightly more obtainable reagents
such as Sulfamic acid, Silica sulfuric acid and Cellulose sulfuric acid with a admittedly lower yield than SBSSA but considering the extremely high
activity of Pancuronium bromide i don't think a low overall yield is going to be to much concern, and maybe even favorable.
http://www.scielo.org.mx/pdf/jmcs/v56n4/v56n4a8.pdf
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Assured Fish  |
| Quote: |
how is the regioselectivity of this reaction controlled,especially for the epoxide on the left ?
|
Good question, to be honest i never even thought about the sterioisomers
|
regioselective is not the same as stereoselective.What I mean is,how will you make sure the piperdine attacks the carbon nearer to the methyl
group(the quaternary center) rather than the other carbon of the epoxide ?
|
|
|
Assured Fish
Hazard to Others
  
Posts: 319
Registered: 31-8-2015
Location: Noo Z Land
Member Is Offline
Mood: Misanthropic
|
|
| Quote: |
regioselective is not the same as stereoselective.What I mean is,how will you make sure the piperdine attacks the carbon nearer to the methyl
group(the quaternary center) rather than the other carbon of the epoxide ?
|
Oh i see, how foolish, well on the acetylated side the formation of the ketone from the ester would cause the selectivity but on the other end i
honestly have absolutely no idea, Fuck.
|
|
|