RogueRose
International Hazard
    
Posts: 1594
Registered: 16-6-2014
Member Is Offline
|
|
Storing CO2 for later use and possible uses in chem
I've done many experiments where CO2 is released in amounts adequate to try to capture and store it for later use (should I find a good use that is).
I don't think any storage method other than keeping it compressed is really viable due to space limitations (maybe fill an air mattress with it, lol
 . I have a couple rotary compressors from old air conditioners, one that is 1/3
hp and another that is 1hp (this one is pretty big for a compressor!) It seems like it would be simple to pipe the CO2 into the vacuum side of the
compressor and feed it into something like an old propane tank, which I have but it has not been cleaned or modified so IDK the conditions of them. . I have a couple rotary compressors from old air conditioners, one that is 1/3
hp and another that is 1hp (this one is pretty big for a compressor!) It seems like it would be simple to pipe the CO2 into the vacuum side of the
compressor and feed it into something like an old propane tank, which I have but it has not been cleaned or modified so IDK the conditions of them.
If anyone has used propane tanks for storage like this, is there anything that needs to be done to make sure there isn't any contaminates like rust
(or other) on the inside?
I was thinking of making some filters like CaCl2 and possibly activated carbon in cannister/pipe filters to dry any gas either coming into the
compressor or into the cylinder.
I would like to do some experiments with making methanol or maybe a CO generator down the road or maybe some other things that use CO2. I know CO2
can be used to make carbonates when bubbling it through hydroxides or oxides but other than that, I'm not sure what I could use CO2 for so I'm open to
suggestions to that.
[Edited on 12-2-2017 by RogueRose]
|
|
|
Heavy Walter
Hazard to Others
  
Posts: 127
Registered: 17-12-2015
Location: Argentina
Member Is Offline
Mood: No Mood
|
|
Hi
Depending where are you based on, CO2 is really cheap and easy to acquire.
Compressed form: for extinghishers, soda making and compressed gas guns.
Solid form: Ice cream stores usually have it and some would sell a bunch of "dry ice".
Recovering and compressing it from your reactions seems cumbersome and even risky if you cannot assure the tank pressure maximum.
|
|
|
A Halogenated Substance
Hazard to Self
 
Posts: 68
Registered: 7-2-2017
Location: United States
Member Is Offline
Mood: Oxidizing due to extended exposure to oxygen
|
|
Quote: Originally posted by RogueRose  |
I would like to do some experiments with making methanol or maybe a CO generator down the road or maybe some other things that use CO2. I know CO2
can be used to make carbonates when bubbling it through hydroxides or oxides but other than that, I'm not sure what I could use CO2 for so I'm open to
suggestions to that.
|
You could always use CO2 to make carboxylic acids from alkylhalides in grignard reactions.
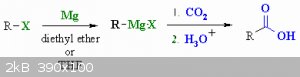
|
|
|
tsathoggua1
Hazard to Others
  
Posts: 335
Registered: 8-1-2017
Location: Beyond the pale
Member Is Offline
Mood: Phosphorescent
|
|
I wouldn't bother attempting to recycle CO2. Its available in small cylinders from any welding store.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
You can store CO2 with bases...
NaOH + CO2 -H2O-> NaHCO3 + Na2CO3
Na2O + CO2 --> Na2CO3
CaO + CO2 --> CaCO3 precipitate
To set it free, only put acid on it...citric, acetic, chlorhydric, sulfuric, ...
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
RogueRose
International Hazard
    
Posts: 1594
Registered: 16-6-2014
Member Is Offline
|
|
Well the thing is, if i'm doing something that is going to generate like 5-15lbs of CO2 (fermentation or making Sodium acetate for baking soda), I
figure I can compress that in a propane tank unless there is something inherently wrong with a propane tank for this.
Long ago in college we had a little 5lb tank of CO2 for a kegerator and it was like $12-18 to fill it depending on which place we went. In town it
was $18, 25 miles away it was $12.. IDK what prices are at welding shops or other places that fill these things (would have to buy the correct
cylinder as well), but I would suspect they are a little cheaper than what we were spending (it was a captive market basically...)
|
|
|
j_sum1
Administrator
       
Posts: 6326
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
If you really are producing that much on a regular basis then my pitch would be for making your own dry ice.
What to do with several kilograms of dry ice you ask??
Ok we are back to square one.
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
True, but your carbon dioxide must be very dry and reasonably pure (unlikely if you're generating it yourself then storing it in an old cylinder).
Using dry ice is far easier and more common when preparing carboxylic acids from organometallic reagents.
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Thermolyse of CaCO3 to CaO and CO2 is another solution in line with my previous proposal.
Alternatively...CaCO3 + solid citric acid, oxalic acid...
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
Using triethanolamine is probably the best otc solution to store and reuse CO2. They are available in soap/cosmetic stores at a reasonable price. At
low temperatures it will absorb CO2 forming a salt, and heating up the solution will release it back. Industrial systems work in that way. The CO2 in
itself is relatively useless besides a few special reaction, tough.
I never asked for this.
|
|
|
j_sum1
Administrator
       
Posts: 6326
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
You might have a process that will allow you to use the CO2 periodically.
For example
I have access to a pool product that contains both lithium and sodium salts. I presume hydroxides since it is alkaline and soluble.
If I had a container of this in solution, any time I was producing excess CO2 I could bubble it through to precipitate lithium carbonate and separate
it from the Na.
No storage needed. Just direct it towards something useful.
You might have something similar that you could use the CO2 for.
|
|
|
urenthesage
Hazard to Self
 
Posts: 77
Registered: 21-2-2016
Member Is Offline
Mood: No Mood
|
|
C'mon man. Baking soda and vinegar for crying out loud. Theres no reason on earth why you'd need to store it.
|
|
|