Quince
National Hazard
   
Posts: 773
Registered: 31-1-2005
Location: Vancouver, BC
Member Is Offline
Mood: No Mood
|
|
Is H2O2 consumed in copper etching with HCl+H2O2?
Or is it just a catalyst in the reaction? And how can I calculate the optimum ratio of acid to peroxide?
\"One of the surest signs of Conrad\'s genius is that women dislike his books.\" --George Orwell
|
|
|
woelen
Super Administrator
        
Posts: 8014
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
H2O2 indeed is consumed initially, but only a limited amount is needed to get the reaction going. In fact, your etching solution improves over time,
when it is used more and more.
Initially, an oxidizer is needed to dissolve any copper metal in the hydrochloric acid. Hydrochloric acid itself is not capable of dissolving copper
metal (see table of electrode potentials). With the help of some oxidizer, however, copper metal can be dissolved. This results in formation of the
tetrachloro cuprate (II) complex. E.g. with H2O2:
H2O2 ---> H2O + O.
O. + 2H(+) + Cu + 4Cl(-) ---> H2O + CuCl4(2-)
The CuCl4(2-) ion is essential to further working of the copper etching liquid. This complex ion is capable of oxidizing copper metal:
CuCl4(2-) + Cu ---> 2CuCl2(-)
The copper (I) complex CuCl2(-) in turn is very easily oxidized by oxygen from the air, provided sufficient acid and chloride is present:
O. + 2CuCl2(-) + 2H(+) + 4Cl(-) ---> H2O + 2CuCl4(2-)
So, once you have some copper (II) in solution, the etching liquid works great, using oxygen from the air as oxidizer. This oxygen is absorbed from
the air extremely easily. So, you first use a small quantity of soluble oxidizer (e.g. H2O2, KClO3) to get the reaction started and once you have
sufficient CuCl4(2-) in solution, you simply use oxygen from the air as oxidizer.
When the liquid becomes very dark at a certain moment, then add a litte concentrated HCl and let it stand in contact with air for a while and then it
is ready to be used for a new batch. Works really great and is very cheap. The waste product is quite interesting as well, it makes fairly pure CuCl2
on evaporation.
EDIT: Fixed error in last reaction equation.
[Edited on 6-12-06 by woelen]
|
|
|
Quince
National Hazard
   
Posts: 773
Registered: 31-1-2005
Location: Vancouver, BC
Member Is Offline
Mood: No Mood
|
|
Thanks.
Should the last reaction have CuCl4 with (2-) instead of (-)?
I assume you can just add a bit of a used solution to fresh acid instead of peroxide, for the CuCl4(2-).
\"One of the surest signs of Conrad\'s genius is that women dislike his books.\" --George Orwell
|
|
|
woelen
Super Administrator
        
Posts: 8014
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Quince, I corrected the last equation. Thanks for pointing me to this error.
A new working solution indeed can easily be made by mixing a used solution with some fresh concentrated HCl.
|
|
|
Quince
National Hazard
   
Posts: 773
Registered: 31-1-2005
Location: Vancouver, BC
Member Is Offline
Mood: No Mood
|
|
woelen, I posted your copper etching equations in an electronics forum and got this reply:
| Quote: | There is only one catch to the scenario you present.
O. + 2 H(+) + Cu + 4 Cl(-) --> H2O + CuCl4(2-)
In order for the gibbs free energy of the above reaction to be negative, the dissolved oxygen concentration must be well above the saturation limit of
oxygen in water. Further, the following reaction has a much more negative free energy than that of the above reaction, meaning that it will take place
preferentially.
O. + 2H(+) + Cu --> H2O +Cu(2+).
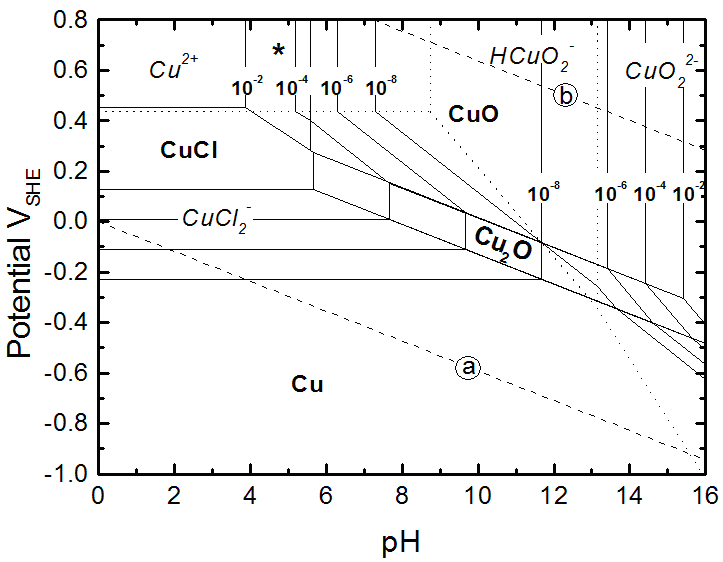
I've attached the Pourbaix diagram for the Cu - H2O - Cl system for your reference. Note that CuCl4(2-) is not present as a stable specie. (Reference:
D. Tromans & R. Sun, Journal of the Electrochemical Society, Vol. 138, pg. 3235 (1991)). Note that the area notated by the "*" in the diagram
represents the area of stability for CuCl2-3Cu(OH)2. |
I've attached the diagram he posted here.
What do you think?
[Edited on 7-12-2006 by Quince]
\"One of the surest signs of Conrad\'s genius is that women dislike his books.\" --George Orwell
|
|
|
woelen
Super Administrator
        
Posts: 8014
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I think that the diagram is not for the conditions, which I mention. This is for low concentration (at most 1 mol / l), while the etching solution is
at much higher concentration of HCl (10 M or so).
But strongest support for my theory is that it actually works. And it works well, very well. Just try it. Dissolve some copper metal in H2O2/HCl mix,
until the liquid becomes dark, at that point copper (I) is formed and all H2O2 is used up.
Now let the solution stand in contact with air. You will see that the dark color disappears quite fast, especially if you shake the thing a little
bit, with contact of fresh air. Then put in some copper-coated PCB and you'll see that it nicely etches, especially if luke-warm.
Replenishment is done simply by leaving the solution stand in contact with air for some time. Every now and then you replace part of the solution
(e.g. 25% of its volume) by fresh concentrated HCl.
Yet another thing. If you have a colorless solution of CuCl2(-) in conc. HCl, then this is amazingly air-sensitive. It really sucks out oxygen from
the air. Have a look at this page, which I wrote about this:
http://woelen.scheikunde.net/science/chem/riddles/copperI+co...
|
|
|
Quince
National Hazard
   
Posts: 773
Registered: 31-1-2005
Location: Vancouver, BC
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by woelen
I think that the diagram is not for the conditions, which I mention. This is for low concentration (at most 1 mol / l), while the etching solution is
at much higher concentration of HCl (10 M or so). |
The diagram goes down to 0 pH, however. Doesn't that imply high acid concentration? Note that I'm not disagreeing, just trying to find which side is
correct. Here's the other thread I pasted that diagram from:
http://www.diyaudio.com/forums/showthread.php?s=&postid=...
[Edited on 7-12-2006 by Quince]
\"One of the surest signs of Conrad\'s genius is that women dislike his books.\" --George Orwell
|
|
|
cabrero
Harmless

Posts: 5
Registered: 1-12-2006
Location: europe
Member Is Offline
Mood: No Mood
|
|
Woelen
In your page's experiment, i think you have at first a soluble and mixed complex of Cu(I)-Cu(II) and Cl-;
In air, perhaps part of the Cu(I) is oxidized to (II).
By hydrolisis it is desestabilized to the oxide-precipitates stables in basic media, part of Cu2O and part of Cu(OH)2.

Now i've seen a few more pages of your site and seems this is also your conclussion;
I've read elsewhere a patent that describes this complex, and uses it to cover a sustrate, that becomes covered by CuOx by inmersing in water.
[Edited on 7-12-2006 by cabrero]
|
|
|