Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
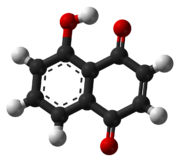
Juglone- From walnut family of trees
https://en.m.wikipedia.org/wiki/Juglone
I collected a bucket of American black walnuts this week, cleaned them up and started the stratifying process in order to plant them next Spring.
Intending to eventually add these trees to the woodlands around our magazines and warehouse, which are largely various 3rd growth and weed trees-
white pine and various other native conifers, European black alder, poplar and birch.
After looking at their main chemical weapon against competeing plant life, I do wonder what else all that juglone might be useful for?

Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
hyfalcon
International Hazard
    
Posts: 1003
Registered: 29-3-2012
Member Is Offline
Mood: No Mood
|
|
Contact herbicide for plant growth suppression? Treat you dogs worms with the tincture also.
|
|
|
Morgan
International Hazard
    
Posts: 1705
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
I just picked up a couple of hundred black walnuts after trimming some branches away from the driveway. It might be interesting to try to dye a shirt
just to see how it would turn out. A few of my fingers have the characteristic persistent stain on them from handling the walnuts with their husks
still on. Here's some I dried a few years ago with the thick husks still on that crenated when dried.
I was at an art fair where a guy sold black walnut wooden spoons that were laser engraved. The guy told me the particular carbon dioxide laser he
bought was expensive. Seems a shame to put the thick branches out by the street to be picked as yard litter.
 
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Can it be synthesized from phenol and maleic acid via the nencki reaction ? https://books.google.co.in/books?id=Wy9P3qCjQBIC&pg=PA15...
|
|
|
Morgan
International Hazard
    
Posts: 1705
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
Some trees and plants sensitive to juglone.
"All species of the walnut family (Juglandaceae) produce juglone. This would include many native trees such as black walnut, butternut, the hickories
and pecan. However, black walnuts have the highest concentration of juglone."
http://www.extension.iastate.edu/news/2005/jul/070701.htm
Some tidbits
Toxicity in Humans
"The active compound in black walnut is juglone. While this substance is found in all parts of the tree, Beverly Shaw, advanced master gardener at
Purdue University says that the fruit, roots and hulls contain the highest concentrations. Apparently, the antimicrobial properties of this natural
chemical also make it toxic. In a study published in Toxicology and Applied Pharmacology on Nov. 15, 2005, authors Michelle T. Paulsen and Mats
Ljungman reported that juglone negatively affects human fibroblasts, specialized cells that reside in connective tissue that produce collagen
proteins. Specifically, the researchers found that juglone drastically decreased available levels of a protein referred to as p53. This event damaged
the DNA material in these cells and triggered apoptosis, or cell death."
"Horses can be affected by black walnut if shavings made from the tree are used in bedding. As little as 20% black walnut in shavings or sawdust can
cause clinical signs within hours of contact. Effects of exposure primarily affect the lower limb and include stocking up, stiff gait, and reluctance
to move. If untreated, toxicosis can progress and cause colic, swelling of the neck and chest, elevated heart and respiratory rate, and even laminitis
and founder. Clinical signs usually disappear once the bedding is removed. The best way to prevent problems is to ensure that bedding does not contain
black walnut."
"Black walnut can cause other problems, although these are reported much less frequently than the two described above. Some people and horses are
especially sensitive to black walnut pollen and can suffer from allergic reactions when pollen is shed in the spring. Additionally, the husks
surrounding fallen nuts can become toxic as they start to decay. Penicillium mold affects the decomposing husk and produces a neurotoxin called
Penitrem A, which is toxic to livestock and can be fatal to dogs. People should also be wary; black walnuts are edible but can be contaminated with
Penitrem A if they hulls have begun to decompose before the nuts are harvested. "
http://www.livestrong.com/article/145740-black-walnut-hull-s...
[Edited on 15-10-2016 by Morgan]
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Well, from an economic standpoint. The Black Walnuts will produce a nice crop while they live, and a whole bunch of money when the trees are
harvested.
That final Harvest might not take place during your lifetime. But, at some time, someone, will be damned grateful for your efforts.
Also interesting, are Chestnut trees. Blight resistant varieties have been developed, and some older varieties do well here in Oregon, where I am
located.
I gifted a pair trees, of the variety Colossus, to friend a while back. Within just a few years, photos came back to me, of the trees covered with
nuts.
What does the structure of Juglone remind me of me of? At first, I thought it was Urushiol, but no. Then, I thought Coumarin, but no. Then,
Hydroquinone, but no. Um, Propofol?
Well, I still don't have it. But, it does remind me of a song.
https://www.youtube.com/watch?v=HFZ3Ed0vHEo
Yer separate parts are not unknown.......
[Edited on 14-10-2016 by zed]
[Edited on 14-10-2016 by zed]
[Edited on 14-10-2016 by zed]
|
|
|
arkoma
Redneck Overlord
      
Posts: 1763
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
Juglone will also paralyze/kill fish in ponds | Quote: | | residents of the American South for easily gathering fish when they threw cut husks into the water with the fish |
quote from here
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
Great dienophile!
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Well, the worm killin' part got me to thinking, about Malaria. But, if Juglone was effective against Malaria, we would probably know about it by now.
There have been some noises, about substituted Juglones however.
Just because Juglone has been known for a while, doesn't mean every potential use has been discovered. The Chinese were using Sweet Wormwood for a
long time. However, absent modern lab testing techniques, folks didn't really suspect that it could actually cure Malaria.
Extensive scientific trials, proved that it could.
https://en.wikipedia.org/wiki/Artemisinin
[Edited on 23-10-2016 by zed]
|
|
|
Dr.Bob
International Hazard
    
Posts: 2748
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
One of my previous coworkers used to harvest a few acres of walnut wood from his family's land every year and would make a substantial amount of
amount. Apparently, some small sawmill in KY would come cut and mill the wood and then sell the entire truck load to a lumber yard in Cal for making
fine cabinets for the wealthy elites there. It produced tens of thousands each year just for a few dozen trees each year, which was only 1% of the
trees per year, so very sustainable. So plant lots of them.
Not sure what juglone is good for, but someone here would likely try to react it with indole to make some spice analogs, I bet.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
I'm also interested into juglone.
Maybe is there a way to convert it:
-by reduction to naphtalene, naphtol, tetrahydronaphtalene, decahydronaphtalene-triol, decahydronaphtalene
-by oxydation to 1,4,5,8-Naphthalenetetrone (and then to tetrahydroxy-naphtalene and then to tetraaminonaphtalene)
Naphtalenetetrone should make a tetraoxime when into contact with hydroxylamine...
-by nitration to dinitrojuglone (I would likely see if it makes an energetic dinitrojuglone dioxime) and finally to trinitrophenol (via side dione
ring oxydation to 4,6-dinitro-2,3-dicarboxy-phenol; then replacement of the 2 carboxy into nitro (2,4,6-trinitro-3-carboxy-phenol and decarboxylation
to TNP)
-by oxydation with HCl and HNO3 to chloranile (tetrachloro-paraquinone) or the naphtalenetetrone variant...chloranile easily exchange its halogens for
azido groups when NaN3 solution is put into contact yielding tetraazidoquinone (azidanile) and it easily exchange its halogen for nitro groups when
NaNO2 solution is put into contact yielding nitranilate (2,5-dihydroxy-3,6-dinitro-para-quinon) (who's lead and silver salts are primaries) via a
transcient tetranitroquinon and partial hydrolysis via nitro-nitrite rearrangement...
R-NO2 <==> R-O-N=O
R-O-N=O + H2O --> R-O-H + HO-N=O
This may be interesting to you:
Attachment: a-new-method-for-the-synthesis-of-juglone-from dihydroxynaphtalene.pdf (168kB)
This file has been downloaded 475 times
[Edited on 27-11-2016 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Nice idea!
I don't know if it would work to the final juglone.
Phenol is activated in ortho and para for the Nencki reaction...and so the first acyl will more likely enter into para position...I honestly don't
know if the second function will react to cyclise.
If the -CO-CH=CH-CO2H suceeds into entering para (1 solution), then the other end would have to react into ortho position vs the group (thus meta vs
the phenolic -OH (two equivalent possibilities))
--> No juglone but an isomer with the OH in position 2 instead of 1.
If the -CO-CH=CH-CO2H suceeds into entering ortho (2 solutions), then the other end would have to react into ortho position (one possibility) vs the
group (thus meta vs the phenolic -OH) --> target juglone.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
I was expecting you might link to this one:
https://www.youtube.com/watch?v=rqEwX9Orp7M
"What is a Jugalone?"
(Well, not quite.)
About that which we cannot speak, we must remain silent.
-Wittgenstein
Some things can never be spoken
Some things cannot be pronounced
That word does not exist in any language
It will never be uttered by a human mouth
- The Talking Heads
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Here is a related tread into the energetic material section.
chrysamminic acid / 1,8-dihydroxy-2,4,5,7-tetranitro-9,10-anthracenedione
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Here is an interesting paper related to juglone and naphtalentetrone ...
[url=https://www.researchgate.net/publication/267958034_OXIDATIVE_DEHYDROGENATION_OF_1-TETRALONES_SYNTHESIS_OF_JUGLONE_NAPHTHAZARIN_AND_a-HYDROXYANTHR
AQUINONES]OXIDATIVE DEHYDROGENATION OF 1-TETRALONES:
SYNTHESIS OF JUGLONE, NAPHTHAZARIN, AND α-HYDROXYANTHRAQUINONES[/url]
Attachment: OXIDATIVE_DEHYDROGENATION_OF_1-TETRALONES_SYNTHESI.pdf (220kB)
This file has been downloaded 475 times
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|