Romain
Hazard to Self
 
Posts: 63
Registered: 23-12-2013
Member Is Offline
Mood: Crystallized
|
|
Synthesis of tris(tert-butyl) alcohol
Hello everyone,
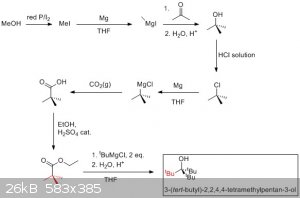
I've been wanting to make the compound mentioned in the title for some time now (see image for the structure and standard name - it's the last
product) because I find its structure interesting and it would be a good exercise to get better lab technique (esterification, grignard, distillation,
etc). The compound is basically tert-butanol with the methyl groups replaced with tert-butyl groups which makes the alcohol function very hindered.
I've been trying to acquire the necessary reagents (namely THF, potassium iodide, red phosphorus, methanol and magnesium) to do the synthesis and
finally got my hands on all of them. So I started experimenting with grignard reagents and I made some tert-butanol this afternoon by reacting
methylmagnesium iodide with acetone.
I thought of a synthetic pathway (see picture) to make the compound I'm after and would like your input on the feasibility. I'm not an expert and I
lack the experience to know if a reaction will work.
Basically I want to convert tert-butanol to the chloride (or bromide or iodide) and make the corresponding grignard reagent. Then I want to react 2
equivalents of it with a tert-butanoate ester (ethyl but any alkyl ester would work I think) to yield tris(tert-butyl) alcohol.
My main concern is the steric bulk in the second grignard reaction, I don't know if it would work.
Any tips/ideas/thoughts/opinions are welcome.
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 827
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
My guess is that the second this hypothetical compound touches acid, it dehydrates instantly. If it would even exist.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4333
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
It doesn't have a beta-hydrogen.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
This compound does in fact exist. Here's an OrgSyn reference for the step you're concerned about,
<url=>http://www.orgsyn.org/demo.aspx?prep=cv1p0524</url>,
looks like it should work just fine.
For the last step however, a quick Reaxys search shows that every reported literature reaction uses t-Butyllithium instead of t-Butylmagnesium
chloride. That might be the step where you run into problems with steric hindrance, and t-Butyllithium is not particularly amateur-friendly to say
the least. There is a relevant JACS paper on this compound which could prove to be a useful alternative, link; be forewarned that the yields are abysmal.
[Edited on 9-15-2016 by gdflp]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4333
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
The comma after the link mucks up the URL.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Whoops, thanks. Fixed.
|
|
|
Romain
Hazard to Self
 
Posts: 63
Registered: 23-12-2013
Member Is Offline
Mood: Crystallized
|
|
Wow didn't even know there was a database like Reaxys! Apparently my uni has access to it so that will probably come in handy in the future.
gdflp: I couldn't see your second link (JACS), SM tells me I'm "not permitted to view this forum". I found a paper maybe it's the same as yours: http://pubs.acs.org/doi/abs/10.1021/ja01615a040 . It describes a prep using t-BuNa but it requires low temperatures (dry ice low).
Anyway I wonder why they use t-BuNa instead of t-BuMgX. As far as I know a grignard reagent is used in a similar way to an alkylsodium.
In any case, I don't think I'll be able to perform reactions with t-BuNa any time soon because of the low temperature required and the need for an
inert atmosphere. Investing in a bottle of inert gas, a vacuum pump and a schlenk line isn't exactly a priority when I only have a water aspirator.
Not amateur friendly as you said.
So to sum it up, the compound can be made, but it too complicated (for me).
I think I'll just stick to a simpler tertiary alcohol for now.
[Edited on 16-9-2016 by Romain]
EDIT: Wrote "Li" when I mean "Na". Fixed.
[Edited on 16-9-2016 by Romain]
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
I posted it in References, sorry about that, I thought you had access. If you have journal access through a uni, here's the DOI: 10.1021/ja01217a049.
|
|
|
Romain
Hazard to Self
 
Posts: 63
Registered: 23-12-2013
Member Is Offline
Mood: Crystallized
|
|
Thanks I found it!
As you said the yield is low unfortunately.
The authors state that the alkyl sodium reagent has more addition power than its lithium or grignard counterpart so that answers my question above as
to why they use lithium.
And they say the product is decomposed by "strong" sulfuric acid solution so I risk decomposing the product during the work up of the grignard
reaction (if the reaction even worked). And then I have to separate it from the by-products...
I think I'll have to abandon my original idea of making this compound... I'll make something else!
Thanks everyone for your help!
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
your concern is justified. Grignard reagents,instead of adding to sterically hindered compounds, end up reducing them.You have to use an organolithium
compound in the last step,as gdflp mentioned.
https://en.wikipedia.org/wiki/Organolithium_reagent#Addition...
what I would have done to make this compound would have been to react pivalonitrile with tert-butylMgBr and then react the ketone formed with t-buLi
[Edited on 16-9-2016 by CuReUS]
|
|
|
Romain
Hazard to Self
 
Posts: 63
Registered: 23-12-2013
Member Is Offline
Mood: Crystallized
|
|
Ah I didn't know grignard reagents could reduce sterically hindered ketones! So there's a definite need for t-BuNa or t-BuLi. Glad I asked before
going any further with my reaction scheme!
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
Quote: Originally posted by Romain  | | Ah I didn't know grignard reagents could reduce sterically hindered ketones! So there's a definite need for t-BuNa or t-BuLi. Glad I asked before
going any further with my reaction scheme! |
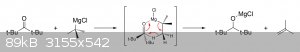
Yes, grignard reagents will often reduce sterically-hindered ketones via a hydride shift or act as a base and deprotonate an adjacent carbon if
alpha-protons are present (giving an enolate that tautomerizes back to the original ketone on protonation).
In your case, the last step of the synthesis would most likely proceed like this:
|
|
|
Cryolite.
Hazard to Others
  
Posts: 269
Registered: 28-6-2016
Location: CA
Member Is Offline
Mood: No Mood
|
|
If you are still interested in making a sterically hindered tertiary alcohol, may I suggest triphenylmethanol? UC235 here has a video on youtube on
its synthesis from ethyl benzoate and bromobenzene, and with the proper glassware it can all be done at home with acceptable yields. You will need to
form the reagent itself carefully, though: phenyl grignards are very fussy with moisture. There is also a preparation on orgsyn, if you prefer text.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4333
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Triphenylmethanol is a cool compound- we made it in first year. The nice thing is that you can make the cation with a bit of strong acid.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Romain
Hazard to Self
 
Posts: 63
Registered: 23-12-2013
Member Is Offline
Mood: Crystallized
|
|
Triphenylmethanol is definitely something I'd like to try but I don't have bromobenzene or benzene, or even benzophenone for that matter. I've seen
UC's video and can only admire his technique and professionalism. Sadly he doesn't seem to make videos anymore.
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
I'd say use a benzoate ester instead, it's easier to come across than benzophenone. If you gather enough benzoic acid then making benzene should be
feasible.
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
I happened to find some benzophenone on eBay for a good price recently (unfortunately just a one-time sale) so I'm going to put out a video at some
point showing the synthesis of triphenylmehtanol using benzophenone and phenylmagnesium bromide. Eventually I plan to take it all the way to
tetraphenylmethane.
|
|
|