Nitrojet
Hazard to Self
 
Posts: 56
Registered: 21-10-2006
Member Is Offline
Mood: No Mood
|
|
Hexanitroethane
According to Urbanski, Hexanitroethane in an admixture with oxygene-deficient explosives such as Tetryl and TNT gives exceptionally powerful HEs. can
anybody please give me the major detonation parameters peculiar to these kinds of explosive mixtures?
|
|
|
Axt
National Hazard
   
Posts: 778
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
http://pdf.aiaa.org/jaPreview/AIAAJ/1963/PVJAPRE1544.pdf
Though its density seems to be debated.
See attachment for a mention of BTF/HNE balanced to CO2. 2.05g/cm3; 9440m/s; 44.9GPa.
[Edited on 22-10-2006 by Axt]
Attachment: developement of high efficiency energetic explosives.pdf (65kB)
This file has been downloaded 2561 times
|
|
|
Nitrojet
Hazard to Self
 
Posts: 56
Registered: 21-10-2006
Member Is Offline
Mood: No Mood
|
|
Thanks for the Pdf files. I studied them thoroughly but unfortunately I did not find any data specifically dealing with the
Hexanitroethane/NitroAromatic mixtures. For in numerous writings it is cited that such mixtures are surprisingly powerful, i prepared afew grams of
Hexanitroethane following the Bromopicrine process. The cumbersome route to this material finally yielded 27gr of the product which then i mixed it
with Tetryl in Stoichiometric ratio. considering the oxygene balances of the materials, -47.4% for Tetryl and +42.7% for HNE, a weight ratio of 50/50
was quite satisfactory to give a zero oxygene balance. I pressed a 40gr mixed sample into a thick-walled polyethylene container under the loading
density of 1.43gr/cc. The charge was primed with 1gr PETN which itself initiated with 2gr of highly purified Mercury Fulminate ( Recrystalized from
Ammonia). The whole assembly was detonated against a thick concrete block for comparison purposes. 8 pieces of lumbers were also put on top of the
charge in an array just to make an indicator of the blast power. The resulting detonation was unbelieving powerful while it shattered the concerete
target into numerous pieces. i recovered some fragments of the wrecked lumbers as far as 250 yards away from the firing point. similar tests with
Pressed PETN ( PETN/WAX 90/10) proved that HNE/Tetryl mixture is significantly more powerful. unfortunately by far i could not find any reliable
laboratory data for the blast and detonation parameters of this class of explosives. is there any reference book which satisfactorily covers the
properties of this mixture?
|
|
|
Axt
National Hazard
   
Posts: 778
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
Do you have the details of the synthesis via bromopicrin?
I've never seen any "numbers" specific to HNE/nitroaromatic mixtures, only that for BTF. That pdf is the first of two pages, you could buy the other
and hope it provides reference to it but I dought it.
http://www.aiaa.org/content.cfm?pageid=406&gTable=japape...
Hexanitroethane patents attached. US3101379 does mention that it forms adducts with aromatics, thus aiding in one of the main issues with
fuel/oxidiser mixtures- homogenicity. GB24839 is the patent for HNE/TNT etc. though no properties listed.
[Edited on 23-10-2006 by Axt]
Attachment: hexanitroethane US3101379-GB24839.pdf (320kB)
This file has been downloaded 1781 times
|
|
|
Nitrojet
Hazard to Self
 
Posts: 56
Registered: 21-10-2006
Member Is Offline
Mood: No Mood
|
|
In a laboratory scale, Hexanitroethane can be synthesized only with great difficulty. Of the many routes suggested for preparation of this compound,
the Bromopicrin process seems to be the most appropriate and hence has been widely practiced for small scale manufacture of the aliphatic nitro
compound, Hexanitroethane. As mentioned earlier, the precursor to this method is bromopicrin itself which can be obtained through distilling a
solution of picric acid with bromide of Lime. The following procedure gives a detailed account of the preparatory method:
Four parts of calcium oxide and 50 parts of water are mixed
in a flask. Six parts of bromine are then added in small portions while the flask is shaken and externally cooled to prevent an excessive rise in
temperature. One part of picric acid is then added and the mixture distilled under reduced pressure. The bromopicrin passes over in the first
fractions of the distillate. It is separated from the water and dried over calcium chloride.
Further treatment of the precipitated product with an alcoholic solution of Potassium cyanide and potassium nitrite gives the second intermediate to
the HNE. This is called Potassium salt of Sym-Tetranitroethane. Dissolving the latter salt in fuming Nitric acid gives the final product. The final
stages of synthesis can be found in details in " Encyclopedia of explosives and related items" by: Basil.T.fedoroff
|
|
|
Nitrojet
Hazard to Self
 
Posts: 56
Registered: 21-10-2006
Member Is Offline
Mood: No Mood
|
|
This photo clearly depict the high blast effect of the Hexanitroethane-Tetryl mixtures. The charge was simply a 40gr mixed sample of HNE-Tetryl in
50/50wt ratio which then pressed into a HDPE container and successfully initiated by a composite detonator with PETN as the base charge and Mercurary
fulminate as the primary. I employed a bridge-wire type firing mechanism for the detonator and the whole assembly was buried 2ft down with a concrete
block in the bottom. blasting cap was then fired with a 12V Lead-acid battery. Photo taken 2 sec after initiation when the shattered lumbers began to
rain down here and there!
Camera 200m off the shot!

|
|
|
Axt
National Hazard
   
Posts: 778
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
I wouldnt say it clearly shows anything, could be ANFO for all we know. Much better to show before 'n after photos of hard targets. I imagine the
performance of hexanitroethane would be simular to that of tetranitromethane mixtures if at simular densities and "homogenicity" (is that even a word
 ). HNE has higher potential density, but thats no good if its not used. ). HNE has higher potential density, but thats no good if its not used.
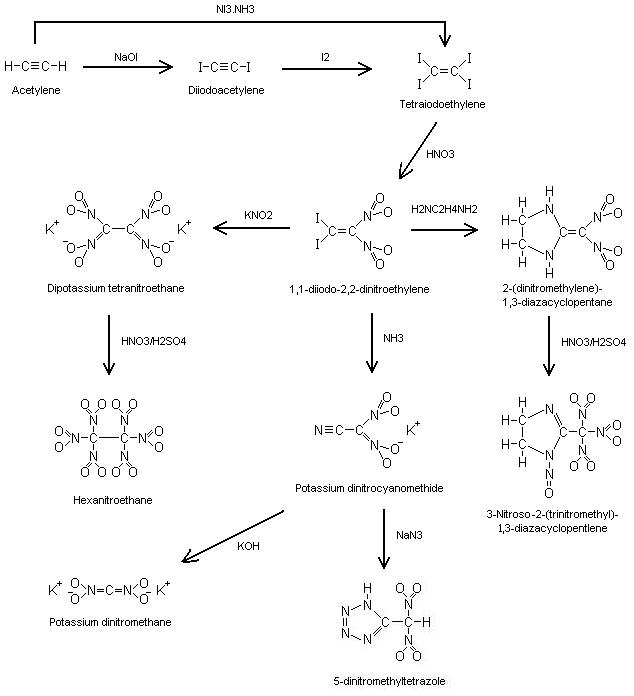
I drew up a scheme starting from <a href="http://www.sciencemadness.org/talk/viewthread.php?tid=4915">diiodoacetylene</a>, this thread
gave me the link between K-tetranitroethane and HNE. Looks easy enough if one possesses iodide salt. I included the production of dinitrocyanomethane
(dinitroacetonitrile) which was the result in the original failed attempt at <a
href="http://www.sciencemadness.org/talk/viewthread.php?tid=5346">FOX-7</a>. Its only stable as its salts.
EDIT: Off topic but I included K-dinitromethane and 3-Nitroso-2-(trinitromethyl)-1,3-diazacyclopent-1-ene.
[Edited on 28-10-2006 by Axt]
|
|
|
Nitrojet
Hazard to Self
 
Posts: 56
Registered: 21-10-2006
Member Is Offline
Mood: No Mood
|
|
This devised synthetic route to HNE sounds really interesting. Although Diiodoacetylene is highly toxic and categorized as a military poison, it can
not be deemed as a set back for the whole procedure as when compared with the Bromopicrin process, equally hazardous conditions prevail. The latter
material is too downright sensitive to all sorts of mechanical stimuli and can explode upon distillation if not carefully heated. it also possesses
powerlful physiological properties and gives out noxious fumes even in relatively low temperatures.
I cheked out "Hawley's Condensed Chemical dictionary" for more information on Diiodacetylen and Tetraiodoethylene. Here is what i found:
Diiodacetylene
CAS: 624-74-8
Properties: white crystals, unpleasant odor. Mp 78.5'C (decomposes). Light acts upon it, causing a gradual change in color to red and a separation of
iodine. Soluble in alcohol, ether, benzene; insoluble in water.
Derivation: By dissolving iodine in liquid ammonia and passing acetylene into the solution
Hazard: Highly Volatile. Toxic by inhalation; vapors irritating to eyes and mucous membranes.
Use: Organic Synthesis, Military Poison!!
Tetraiodoethylene
Properties: Light-yellow crystals, odorless, turns brown on exposure to light. Mp 187'C, d 2.98. Insoluble in water, soluble in most organic solvents.
Derivation: Idonine on Diiodacetylene
Do you have any details of synthesis following Diiodacetylene procedure?
|
|
|
Axt
National Hazard
   
Posts: 778
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Nitrojet
Do you have any details of synthesis following Diiodacetylene procedure? |
Yep, its all available in the literature, I just put the jigsaw together. All the intermediates are likely toxic, but probably less so then
hexanitroethane itself! so I guess if thats the aim your gunna have to be careful anyway.
See the attachment for all the journal articles detailing each step.
EDIT: Included the dinitromethane and 3-Nitroso-2-(trinitromethyl)-1,3-diazacyclopent-1-ene references.
[Edited on 28-10-2006 by Axt]
Attachment: acetylene-hexanitroethane.zip (4.4MB)
This file has been downloaded 1104 times
|
|
|
Nitrojet
Hazard to Self
 
Posts: 56
Registered: 21-10-2006
Member Is Offline
Mood: No Mood
|
|
I think Hexanitroethane is not that much poisonous. It is true however that extreme care should be taken to avoid skin contact. Same as
Tetranitromethane this material can cause seroius and developed form of "Methaemoglobinaemia". But due to much less vapor pressure, it is inferior to
TNM regarding toxicity. Wearing protective gloves and avoiding inhalation of dust or accumulated vapors can effectually reduce the poisonousness of
this material. In my laboratory HNE has never caused any kind of Physiological effects. But it simply does not mean that it is not potentially
harmfyl.
Special thanks for your Pdf pieces of your jigsaw!
|
|
|
Mason_Grand_ANNdrews
Hazard to Self
 
Posts: 63
Registered: 19-1-2006
Location: New Berlin City !!
Member Is Offline
Mood: crabby
|
|
Thans to download the PDF`s, some nice ideas. I have never
heard about hexanitroethane. I would think
hexanitroethane is extremely sensitive and the synthesis is
somewhat dangerous, when possible. I have searched
around the web about hexanitroethane but nothing to
found. I don`t know if that can right. May be anyone can
test this out. It would safe when you use a vacuum
equipment by handling nitromethane and HNO3, a vacuum
pump is sufficient. I belive, a nitration of nitromethane with
concentrated HNO3 or H2SO4 will give maximum 1,1,2,2-
tetranitroethane. Such a process will generate a lot of heat
and the mix can explode. More a problem is to found
somewhat to the boiling points of the nitroethanes, that
they are soluble in water and if the acidic nitroethanes are
stable. Are you shure that the boiling points are correct,
hexanitroethane 150 °C ?
I have coosen some nice suggestions to obtain nitroethanes:
Nitroethane:
Prepare a cilled solution of 20 g of bromoethane, 13 g of
powdered sodium nitrite and 500 ml of dimethylsulfoxide in
a 1000 ml beaker. Seal the beaker with glass wool, set the
beaker to hot water bad and heat up the water bad for an
hour to 100 °C. Let cool somewhat, remove the glass wool,
heat for additional 30 minutes to remove the remainders of
bromoethane, nitrogen oxides and HNO3. Set the beaker to
a oil bad, connect the beaker to a distillation equipment,
remove the air with a vacuum pump and the nitroethane is
then careful distilled between 114 and 116 °C. The product
contains some remainders of DMSO and can be purifyed in by
distillation. It is possible to use chloro- or iodoethane
instead bromoethane.
Dinitroethane: (1,2-Dinitroethane)
Place 5 g of nitromethane to a 500 ml beaker, cool the
beaker to 0 °C and careful add 30 ml 35% HNO3 (70% HNO3
is diluted with water, ratio 1:1). Seal the beaker with glass
wool set the beaker to a oil bad and heat the the mixture
over a period of an half hour to 60 °C. Over a peroid of 30
minutes drop 156 ml of 96-98% H2SO4 to mixture and
shake the beaker somtimes. The obtained dinitroethane is
then filtered or extracted and vapoized under vacuum. The
temperature should maintained exactly, a external cooling
bad will help.
Tetrabromoethane: (1,1,2,2-Tetrabromoethane)
Prepare a mixture of 50 g of bromoethane, 1 g iron powder
and 284 g of bromine in a large 2000 ml beaker. Seal the
beaker with glass wool and heat the mixture over an half
hour to 50 °C and than relux for an hour and rise the
temperature slowly to 230 °C. Let cool the beaker, remove
the glass wool and the remainders of bromine is evaporated
(bp around 58,8-59 °C). Filter the liqiud to remove
ramainders of iron and the tetrabromoethane is then
distilled above 239 °C. The product contains some
remainders of di- and tribromoethane.
Tetranitroethane Synthesis: (1,1,2,2-Tetranitroethane)
Synthesis 1:
Place 1 g of nitromethane to a 100 ml beaker which is
standing in a salt ice bad at 0 °C and over a period of 15
minutes drop 6,5 ml of 70% HNO3 to the beaker. Hold the
temperature below 10 °C and shake the beaker sometimes.
Seal the beaker with glass wool set the beaker to a oil bad
and heat the the mixture over a period of an half hour to 20
°C. Over a peroid of 30 minutes drop 50 ml of 96-98%
H2SO4 to mixture and shake the beaker somtimes and take
care that the temperature is not rise to much. The obtained
the tetranitroethane is then careful vaporized from the
solution under vacuum.
I don´t know the temperatures are correct and the reaction
will not form a pentanitrate, the liqud should not explode
during the addition of the acid. I belive the reaction fails
when to much heat is generated. May be it will work when
nitroethane is dropped to a 0 °C chilled mixture of HNO3 and
H2SO4 and the temperature is hold for any minutes. I don`t
know somewhat about the bp of tetranitroethane but it can
shurely filtered or extracted and vaporized under vacuum by
careful heating up the mixture in a water or oil bad to obtain
the product.
Synthesis 2:
I would guess it is correct when the tetranitroethane is
prepared like nitromethane instead of concentrated HNO3
and H2SO4 with a surplus of DMSO. You can use a mixture
of 200 ml DMSO, 4 g of tetrabromoethane and 4 g of
powdered sodium nitrite.
Hexanitroethane Synthesis:
Synthesis 1:
I think it is real difficult to make hexanitroethane but may be
it will work when tetrabromoethane is nitrated with
HNO3/N2O5 instead HNO3/H2SO4 to 1,2-dinitro-1,1,2,2-
tetrabromoethane and than the product is obtained like the
upper synthesis with 5 g of the obtained product.
Prepare a nitrating solution of 17 g 98-99% HNO3 and 13 g
dinitrogen pentoxide in a salt ice bad at 0 °C. Slowly add 4
g of 1,1,2,2-terabromoethane and the mixture, seal the
beaker with glass wool, stir the mixture for one hour below
10 °C and the obtained product is filtered or extracted and
the 1,2-dinitro-1,1,2,2-tetrabromoethane is than careful
vaporized under vacuum with a oil bad.
Synthesis 2:
May be hexanitroethane can prepared by stirring 0,9 g of
nitromethane in 17 g 98-99% HNO3/13 g dinitrogen
pentoxide for 2 hours below 10 °C.
I would guess a useful idea is to nitrate 1,2-dibromoethane
or 1,2-dichloroethane. Have someone any suggestions to
get the nitroethanes free of acid ?
[Edited on 29-10-2006 by Mason_Grand_ANNdrews]
[Edited on 29-10-2006 by Mason_Grand_ANNdrews]
|
|
|
Nitrojet
Hazard to Self
 
Posts: 56
Registered: 21-10-2006
Member Is Offline
Mood: No Mood
|
|
Hexanitroethane is a colorless crystalline solid which actually melts at 147’C. It is not particularly hazardous upon handling or use. Unlike
Tetranitromethane it is of low volatility while its vapor pressure at 30’C is reported to be 0.8 millibar. Regarding explosive properties it is
known to be of relatively low strength. The corresponding value for lead block test of this explosive stays at 245cc/10gr which is comparable to that
of dinitrotoluene. Its sensitivity to mechanical stimuli is far less than Picric acid. Due to its exceptionally high oxygen content (+43%) it can be
used in admixture with a variety of combustibles to produce brisant explosives. The best results can be attained when HNE is mixed in stoichiometric
ratios with oxygen-deficient explosives of the Nitroaromatic family. It can be directly introduced into a bulk of molten trinitrotoluene to give a
dense cast HE, exceeding nitroglycerine in explosive power. Similarly it can be mixed with Tetryl in powder form and then pressed into pellets to be
used as booster, or any other desirable shape for demolition purposes. I prepared this material only with immense difficulty and following the process
using Bromopicrin as the precursor. The synthesis is potentially dangerous both in view of the toxic nature of the intermediate chemicals and also
explosion hazard. My experiments with HNE revealed that the danger of getting poisoned with this chemical can be effectively prevented if skin contact
and dust inhalation both are strictly avoided. Long ours of working in a small laboratory in which a sample of Hexanitroethane was exposed to ambient
air for drying caused no headache or any other form of poisoning symptoms. On the other hand the explosive power of HNE-Tetryl mixtures is quite
unrivaled when compared with any other explosive I have ever tested. Yet, however, the major drawback to this type of explosives is unavailability of
an effective synthesis route to HNE in a cheap and easy way. Your devised preparatory methods sound very interesting, though the final confirmation
has to be rendered in lab. Nowadays I’m so busy working on Sorguyl, later on I will definitely have some more experiments for HNE synthesis based on
the suggested methods by the readers.
|
|
|
Mason_Grand_ANNdrews
Hazard to Self
 
Posts: 63
Registered: 19-1-2006
Location: New Berlin City !!
Member Is Offline
Mood: crabby
|
|
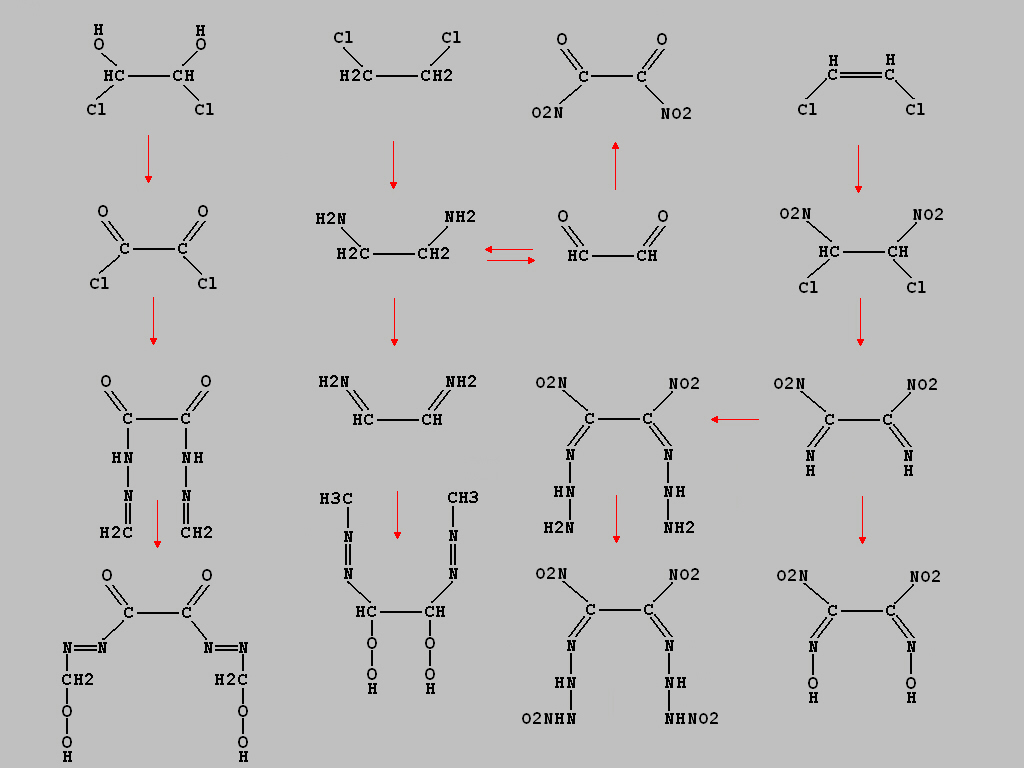
TNX for you expierience of HNE and some infos to the attributes compared to other explosives. I`ve designed a
image to some new nitroethanes related to some metioned
syntheses in the topic n-subtituted amines. I hope my
suggestions looks interested. The compounds should be
producible from 1,2-dichloroethane and 1,2-dichloroethylene.
I don`t have a useful synthesis at the moment to one of the
compounds. The image contains some dioximes, nitramides
within secondary amines and imines as well as two
diazonium compounds within a peroxide. It is not known if
the explosives are stable and actually to use. Hope that
helps and i have something more in the next days. Excuse
me when the image is a little bit to large, it`s somewhat
difficult to convert.
[Edited on 30-12-2006 by Mason_Grand_ANNdrews]
|
|
|
Nitrojet
Hazard to Self
 
Posts: 56
Registered: 21-10-2006
Member Is Offline
Mood: No Mood
|
|
Continued with my experiments with HNE, the aliphatic nitro compound was used as the key explosive-oxidizer part of some blends with various
explosives and non-explosive combustibles. At the very first attempt, Tetryl, an oxygen-deficient candidate from the nitro aromatic family of
explosives was mixed with HNE in stoichiometric ratio. Both of the two components were in the shape of a very fine powder, which then thoroughly mixed
and pressed. Indications made by steel plate dent tests put the resulting binary explosive in the category of highly powerful explosives such as PETN
(HNE/Tetryl is roughly 20% more powerful than PETN). This formulation also found to be only slightly more sensitive to impact when compared with
Tetryl tested under the same conditions. No significant loss of weight was observed when a 2gr sample heated at 65’C for a week.
Of the numerous non-explosive combustibles, atomized aluminum came first on the agenda. A HNE/Al 2/1 Wt. % gives a perfect oxygen balance. Such a
mixture proved to be twice as sensitive as PETN under impact tests. A 20gr sample was prepared and pressed. Upon detonation with a no.6 blasting cap,
it produced a highly powerful shockwave accompanied by a substantial damage to the nearby masonry. This mixture also possesses a great incendiary
effect whilst 10-20gr samples set bunches of dried hay afire at the max. Distance of 8ft. Replacement of Magnesium with Al found to be of no special
benefit. HNE/Al mixtures were also of distinctly less brisance vis-à-vis HNE/Tetryl. (Plate Dent Tests)
The second successful attempt with non explosive ingredients was made with Hexamine. I had a previous account of powerful AN/Hexamine mixtures being
considerably more powerful than the ordinary ANFO. It emanates from the fact that Hexamine is a high-energy fuel possessing a better oxygen balance
(-205%) than Kerosene or Diesel Oil. So it makes possible to increase the fuel ratio almost twice as much as the maximum fuel oil level in an AN
explosive. I got the best results with AN/Hexamine 90/10. HNE/Hexamine mixtures come into zero balance with the weight ration of 5/1 HNE/Hexamine.
Provided that the fuel is of very good quality, such a mixture is a surprisingly powerful explosive. The impact sensitivity values resemble those of
Picric Acid. Detonation of 10gr sample on cold rolled steel plates (13mm in thickness) caused a marked impression. The latter formulation is more
brisant than the Aluminized HNE. It can be attributed to the much higher gas yield while addition of aluminum reduces the gas volume in favor of the
greater heat of detonation. Of particular interest can be HNE-Ethyl Nitrate Mixtures which remains to be prepared and tested. Excellent results might
be attainable if Ethyl Nitrate acts as a solvent towards HNE. Nevertheless the resultant solution can be of poor chemical stability.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
I am unaware that 1,2diiodoethylene (diiodoacetylene) is regarded as a military poison.
But tribromonitromethane (bronopicrin) most certainly is.
In fact EPA regards it as more of a threat to public health than chloropicrin, as it arises as a product of chlorination of drinking water.
Suffice it to say neither one is a walk in the park with your favorite girl.
BTW Federoff's is chock full or errata, not all of which were corrected in later volumes.
|
|
|
Mason_Grand_ANNdrews
Hazard to Self
 
Posts: 63
Registered: 19-1-2006
Location: New Berlin City !!
Member Is Offline
Mood: crabby
|
|
Is an amin chlorinateable ?
I can`t answer your question because i don`t have
knowledge what the militarys use in their applications.
I have a nice idea to get a useful nitroethane within an
nitramin in this topic, for instance
1,2-dinitoethane-1,2-dinitramin
(DNEDNA, O2NHN)(O2N)2C-C(NO2)2(NHNO2) ) give a
somewhat stable seconary explosive.
My idea is to prepare first the
1,2-dinitroethane-1,2-dinitroamin by the way of
1,2-dichloroethylene.
1,2-dichloroethylene ---> 1,2-dichloro-1,2-dinitroethane ---> 1,2-diamino-1,2-dinitroethane
or
1,2-dichloroethylene ---> 1,2-diaminoethylene ---> 1,2-diamino-1,2-dinitroethane
by dissolving the dichloride in for instance in enough
anhydrous ethanol and bubbling anhydrous ammonia
into the liquid until the molar weight is increased. My
question is, is an amin chlorinateable
when the result of 1,2-diamino-1,2-dinitroethane is strong
chlorinated ? Does someone have any infos to
the technique beside other more costly methods to
chlorinate an amin. It can happens the result is a mixture of
1,2-dinitroethane-1,2-dichloramin and
1,2-dichloro-1,2-dinitroethane-1,2-dichloramin.
1,2-diamino-1,2-dinitroethane ---> 1,2-dinitroethane-1,2-dichloramin ---> 1,2-dinitroethane-1,2-dichloramin
ClHN-O2NCH-CHNO2-NHCl
I`m shure the purifyed product of
1,2-dinitroethane-1,2-dichloramin is then aminated to by the same solvent(s) to the secondary amin.
(H2NHN)(O2N)CH-CH(NO2)(NHNH2)
My next question is, an amin can transformed to an nitrate in a stron acid environment of
H2SO4(SO3) and HNO3 99%+ . Could this work with an amin or seondary amin, something risky but an decent performed
synth. should give a useful quantity of an nitramin.
(O2NHN)(O2N)CH-CH(NO2)(NHNO2)
The final product contains a mix of different isomers of
1,1,2,2-tetranitoethane-1,2-dinitramin, (O2NHN)(O2N)CH-CH(NO2)(NHNO2)
and
1,2-dinitoethane-1,2-dinitramin, (O2NHN)(O2N)2C-C(NO2)2(NHNO2)
Separation should be easy  . Please commented guys and some infos are helpful. . Please commented guys and some infos are helpful.
Please excuses the bad quality of the picture, no good drawing software available.
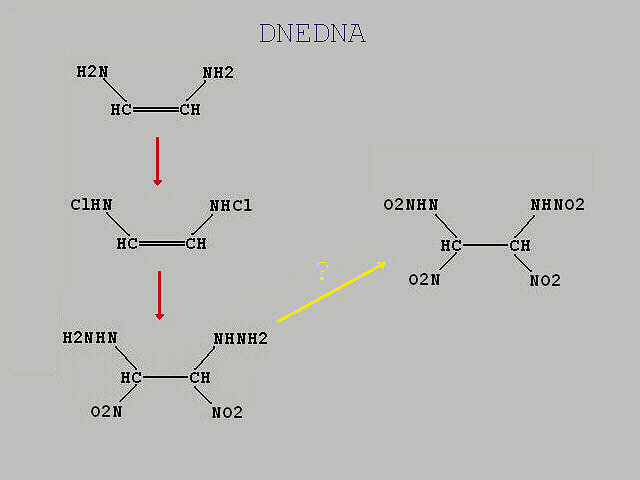
|
|
|
Axt
National Hazard
   
Posts: 778
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
Mason...ect. Why are you posting this into this thread. Everything you've posted is a load of crap and any response to it can only push this further
off topic.. Maybe you should make a topic of your own and call it "Mason_Grand_ANNdrews crazy synthesis of impossible molecules" or words to that
effect, and i suggest u start it in detritus.
Crap crap crap.
[Edited on 16-5-2007 by Axt]
|
|
|
Mason_Grand_ANNdrews
Hazard to Self
 
Posts: 63
Registered: 19-1-2006
Location: New Berlin City !!
Member Is Offline
Mood: crabby
|
|
Excuseme Guys, the treat is going on a litle bit longer than
crap can ever be, because of i`ve sometimes far from the
material energetic knowledge and in this "simple" chemical
science. But i know, the knowlede is not easy bu what should i
do when “your forgotten ideas" coming back. I know my latest
pictues looks like a little bit simple and simetimes
like a shitty "mona lisa" Not every day a useful "Gespinnst" is
comming down to will be a poem to thinking far away from "real
useful" Something is "wow" and is a useful crappy darkness of
ideas. Why not more of this ? Suggest is "rar"
and snth. way to this more than missing. Bleating is easy but a
little bit more than give me a hint will be a way of "sing my
songs". Why should you not do what you want. A picture can
help to desing a world of own business for you and not for
the ""rest of the world"". When you found a great job of the
snth. of energtic msterials, do it. Nobody can prevent you.
Now a simple synthesis to my last pic and he snth. of
1,2-dinitoethane-1,2-dinitramide.
I hope it will work. It`s a suggestion only, I don`t have access
to the database of more than “simple prepared” nitramides.
Synthesis 1:
Add drop by drop some of fresh destilled formamide to a
mixture of some ml of fresh destilled formamide with hexamtylenetrtamine ans paraforaldehyde, stir until all is mixed and the solution is not heated
to much. Seal it in a adequate flask for 14 days, shake it sometimes and than the mix is poured over a acid filter. Maybe it works when the mix is
heated for some hours over the dp of the hexamine.
I don`t know it`s necessary to clean the solution free of acid
when it nitrated with a little waste of acetic acid anhydride and > 99% HNO3, the solution contains remainders of formamide, formic anid and H2O.
Prepare a nitrating mixture of 99% HNO and fresh prepared
acetic acid anhydride in a adequate beaker (approximatly ratio 1:4), set the beaker in a ice water bad and careful stir in excuse me the “acetic
diformamide solution” Shake the beaker
sometimes rapidly and let the solution reakt for two hours.
Crystals of 1,2-dinitoethane-1,2-dinitroamide should formed
which can be filtered and washed with some distilles water or a solvent which is not dissolve the stuff to much. Excuse me it`s only a
philosophy of possibility and was not tested by me, why notTo store or purify the material it must be dissolved
maybe in formic acid  and keep it free of remainders of acid. and keep it free of remainders of acid.
Synthesis 2:
More than interested seams, will the way work when an simple
–NH3 is chlorinated in the presence of red phosphorus and sun
light ? Why should it not possible with the easy way of bromine ?
I know the way of a alpha chlorination of a –CH3 group
works. Why not with an amin ?
I think a real possibility to prepare a mixture of 1,2-dinitoethane-1,2-dinitramide and futher stuff is as follows:
A calculated amount of 1,2-dichloroethane is nitrated by for
instance 98.99%HNO3 and acetic acid anhydride or
HNO3/H2SO4 to 1,2-dinitro-1,2-dichloroethane, is purifyed and
than treated with NH3 gas or ammonium hydroxide to
1,2-dinitro-1,2-diaminoethane. The somewhat purifyed stuff
(maybe by distillation on the bp) is than mixed with calculated
quantities of bromine and red phosphorus powder in a waste of
CCl4 (I don`t know the solvent will work correct) in a adequate
flask and the slurry is than careful stand in a warm water bad
for some days in the sunshine. The flask should be sealed with
a fine glas wool stopper. To complete the reaction heat the
beaker for some hours is a hot water bad and the liquid is
careful distilled over in a equipment with a vacuum pump to
separate it from the red phosphorus.
The result should be a mix of
1,2-dinitro-1,2-diamino-1,2-dibromoethane[(NO2-Br-NH2)-C-C-(NH2-Br-NO2)]
and
1,2-dinitro-1,2-dibromoethane-1,2-dibromoamin
[(NO2-Br-NHBr)-C-C-(NHBr-Br-NO2)].
Keep the temperature careful that all can distill over and the
liquid is not explode. The chilled material is than converted with
the known methods to 1,2-dinitro-1,1,2,2-tetraaminoethane
(NO2)2(NH2)4-C2
and
1,2-dinitro-1,2-diamino-1,2-dinitroamin
(NO2)2(NH2)2-(NHNO2)2-C2
Excuse me for the easy suggestions but I think it`s easy to
calculate the quantities of the reactions but i don`t have time
for this at the moment. I know it exist more than one of this
possibilities, but it needs much more time to came up with some
of the suggestions for this stuff. Maybe someone can test it
out ?
[Edited on 26-9-2007 by Mason_Grand_ANNdrews]
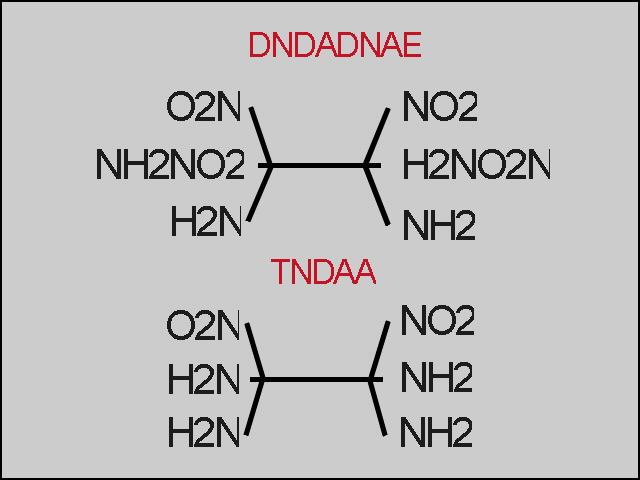
|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
What about action of KNO2 (or better AgNO2) on hexachloroethane C2Cl6 in methylene chloride? Will it work? If not, why?
[Edited on 1-10-2007 by Engager]
|
|
|
Mason_Grand_ANNdrews
Hazard to Self
 
Posts: 63
Registered: 19-1-2006
Location: New Berlin City !!
Member Is Offline
Mood: crabby
|
|
TNX for he hint. I will look for more of this. My Knowledge is not adequate at the moment. Excuse me for the "little" error im my last post.
Add drop by drop some of fresh destilled formamide to a
mixture of some ml of fresh destilled concentrated acetic acid with hexamtyleneteramine and paraforaldehyde, stir until all is mixed and the solution
is not heated to much. Seal it in a adequate flask for 14 days, shake it sometimes and than the mix is poured over a acid filter. Maybe it works when
the mix is heated for some hours over the dp of the hexamine.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Tin Chloride / Sodium Nitrite as a New Nitrosating Agent for N-Nitrosation of Amines, Amides and Ureas
http://www.thieme-connect.com/ejournals/abstract/synthesis/d...
A patent on obtaining dinitroalkanes from nitronate salts using sodium nitrite
http://72.14.205.104/search?q=cache:-ruFGC2ad80J:www.patents...
What's interesting in this process is the possibility of obtaining 2,2-Dinitro-1,3-propanediol
( shown here below ) from nitromethane and formaldehyde see - example 4 - in the above patent
This can then be further nitrated into dinitro-propane dinitrate (CH2NO3)2=C(NO2)2 ->3 CO2 + 2 H2O + O2
http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=7595...
[img]http://pubchem.ncbi.nlm.nih.gov/image/imgsrv.fcgi?t=l&cid=75955[/img]
[Edited on 27-10-2007 by franklyn]
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
"Bad gateway"! Please give the correct URL.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Hexanitroethane
Hexanitroethane is a colorless crystalline solid which melts at 147 degC*, with significant decomposition. It is not as hazardous to handle as
tetranitromethane because of its low volatility. Hexanitroethane has a vapor pressure of 0.8 millibar at 30C. However, the toxicity between the two
compounds is otherwise similar for a given concentration in air. Hexanitroethane can give more powerful explosive mixtures with nitroaromatics than
tetranitromethane because of its higher density. Hexanitroethane is not thermally stable. At 70 degC, the half-life is about 400 hours. The
decomposition rate is 10-20 times faster if dissolved in a hydrocarbon solvent. The measured density for Hexanitroethane is 1.85g/cm3. A more accurate
measure of the maximum density is problematic because melting the compound results in partial decomposition, and it is difficult to completely
separate the solvent if crystallization is attempted, as evidenced by an altered (slightly lower) melting point. For the above mentioned measurement,
the loose hexanitroethane immediately obtained from the synthesis was put into a high-pressure mold to form a more compact solid and exclude air.
*this is the melting point according to the original source, which may have been influenced by traces of unremoved solvent. Wikipedia gives a
different melting point at 135 °C.
Synthesis Route
The potassium salt of 1,1,2,2-tetranitro ethane was treated with concentrated sulfuric and nitric acids dissolved in methylene chloride, which
produced hexanitro ethane C2(NO2)6 in 92% yield.
The potassium salt of 1,1,2,2-tetranitro ethane could possibly be obtained by reacting glyoxime with a solution of sodium nitrite, NaNO2, and nitrogen
dioxide.
Quote: Originally posted by Axt  |
The activated hydrogen of glyoxime is capable of reacting with nitric acid containing HNO2 or with nitrogen dioxide itself yielding nitroglyoxime, an
explosive nitrolic acid.
E. Bamberger & U. Suzuki, “Űber Nitro-Glyoxim” Berichte der Deutschen Chemischen Gesellschaft, 45, 2740-2758, (1912)
Fedoroff, B. et al. “Encyclopedia of Explosives and Related Items“. vol. 6 pg. G119. (1974)
|
Ethyl nitrolic acid, was prepared by the action of potassium hydroxide and sodium nitrite on nitroethane (Meyer & Constam, 1882). It develops a
bright red color in alkaline solution. The compound has two structural tautomers:
the aci-form, CH3CH2(=NOH)-NO2, and
1-nitro,1-nitroso-ethane, CH3-CH(-NO2)-N=O
Nitroso groups can usually be oxidized to nitro groups using dilute H2O2. It would probably be better to first isolate the ethylnitrolic acid, before
oxidation, by neutralization with dilute HCl, then extraction into an oil solvent.
1,2-dinitroethane can be prepared by reacting NO2 with ethylene at room temperature, in the absence of oxygen. The dinitroethane could then
potentially be used as the precursor to 1,1,2,2-tetranitroethane in the same way that nitroethane can be used to make 1,1-dinitroethane described
above. To form the salt, simply react the tetranitroethane with dilute potassium hydroxide.
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Quote: Originally posted by Axt  | I wouldnt say it clearly shows anything, could be ANFO for all we know. Much better to show before 'n after photos of hard targets. I imagine the
performance of hexanitroethane would be simular to that of tetranitromethane mixtures if at simular densities and "homogenicity" (is that even a word
 ). HNE has higher potential density, but thats no good if its not used. ). HNE has higher potential density, but thats no good if its not used.
I drew up a scheme starting from <a href="http://www.sciencemadness.org/talk/viewthread.php?tid=4915">diiodoacetylene</a>, this thread
gave me the link between K-tetranitroethane and HNE. Looks easy enough if one possesses iodide salt. I included the production of dinitrocyanomethane
(dinitroacetonitrile) which was the result in the original failed attempt at <a
href="http://www.sciencemadness.org/talk/viewthread.php?tid=5346">FOX-7</a>. Its only stable as its salts.
EDIT: Off topic but I included K-dinitromethane and 3-Nitroso-2-(trinitromethyl)-1,3-diazacyclopent-1-ene.
[Edited on 28-10-2006 by Axt] |
I think this picture is interesting since the proceedure isnt too complicated, and this CN3O6 seems very oxygen positive oxidizer compared to other
oxidizers while being gas less, and producing NO water so 100% no smoke or steam.
While the synthesis is not hard, is it possible for the same reaction to be carried out with TetraCHLOROethylene instead of TetraIODIethylene because
the chloro one is much more widely sold and easy to obtain.
Also at interest of the salt - K2+ [C(NO2)2]2-
I guess it could process this ion exchange.
K2+ [C(NO2)2]2- PLUS NH4+ NO3- Becomes K+NO3- and (NH4)2+[C(NO2)2]2-
(NH4)2[C(NO2)2]2
This could be interesting, it is 100% gaseous while having an oxygen balance of Zero.
(NH4)2[C(NO2)2]2 >Detoanates> = 2CO2 + 4H2O + 3N2
Just some guesses.
|
|
|
|