| Pages:
1
2 |
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
Good news from Dr. Nichols: 1-Aminomethylbenzocycloalkanes
1-Aminomethylbenzocycloalkanes: Conformationally Restricted Hallucinogenic Phenethylamine Analogues as Functionally Selective 5-HT2A Receptor
Agonists1
McLean, T. H.; Parrish, J. C.; Braden, M. R.; Marona-Lewicka, D.; Gallardo-Godoy, A.; Nichols, D. E.
J. Med. Chem.; (Article); 2006; 49(19); 5794-5803. DOI: 10.1021/jm060656o
http://rapidshare.com/files/79842/New_Nichols.pdf.html
The route to the compound 2 seems rather tedious. To get the key precursor, I was having in mind that one can take allylbromide,
react with potassium phthalimide to get the masked allylamine and let this undergo a [2+2] photoaddition with benzoquinone under standard conditions,
for conveniance... Ideas?
Anyways, tnx to such a cute girl for showing me the paper... 
[Edited on 22-10-2006 by Sandmeyer]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Do you happen to have any useful references for 2+2 photoadditions where a terminal non-conjugated alkene is photocyclisized with p-benzoquinone?
It simply sounds too simple to be true, so I'm very skeptical about it.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Sergei_Eisenstein
Hazard to Others
  
Posts: 290
Registered: 13-12-2004
Location: Waziristan
Member Is Offline
Mood: training
|
|
In theory it' possible, but I'm more sceptical about "standard conditions". Reaction conditions for pericyclic reactions vary widely, from -50°C to
280°C and from 1 to 100 atm.
Things that should be considered: are you sure the HOMO/LUMO energy difference is large enough to drive the reaction into the desired direction (the
olefin is not very activated, as Nicodem pointed out)? You may instead end up with Paterno-Büchi side-products or photodimerized allylamine (gives a
symmetrical 1,3-disubstituted cyclobutane). Also, if you obtain the desired intermediate, how easy (how many reaction) will it be to have the
aromaticity of the new benzoquinone derivative restored? It's not as simple as the reduction of benzoquinone to hydroquinone (as far as my knowledge
reaches).
damnant quod non intelligunt
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
Hi 
Thanks for the input... Sorry but I don't have p-benzoquinone-specific references, I posted what came to mind while looking at the formulas  . However, 3-methyl-2-cyclohexenone reacts smoothly with
ethylene[1] as does plain cyclohexenone (90% yield), undesired Paterno-Büchi heterocycle is formed only in 3%
yield[2]. Another procedure[3] uses electron-rich olefin (note also interesting fragmentation). I did consider
acroylnitrile, but wouldn't the use of isolated alkene minimize side-reactions (dimerizations), since in that case only enone can be light-excited? I
see no frontier orbital obstacle to have isolated olefin as one of the components. . However, 3-methyl-2-cyclohexenone reacts smoothly with
ethylene[1] as does plain cyclohexenone (90% yield), undesired Paterno-Büchi heterocycle is formed only in 3%
yield[2]. Another procedure[3] uses electron-rich olefin (note also interesting fragmentation). I did consider
acroylnitrile, but wouldn't the use of isolated alkene minimize side-reactions (dimerizations), since in that case only enone can be light-excited? I
see no frontier orbital obstacle to have isolated olefin as one of the components.
Anyhow, another option is to go via aryne corresponding to 2-bromo-p-dimethoxybenzene. Once generated, this species
a.) may be able to undergo a [2+2] with, N,N-dibenzylallylamine* or N-allylphtalimide** to give protected amine directly
b.) may for sure undergo a [2+2] with 1,1-dimethoxyethylene, ultimately giving cyclobutanone[4]. Treatment with
TosMic yields nitrile. But b.) involves much more work...
*: If BuLi is used since it will cleave the phthalimide
**: If LDA is used since it dosen't attack phthalimide
Nichols et al. use 3 different starting materials in Scheme 1, 2, 3, more efficiently one could go from indanones to make both five-membered (IMO,
TosMic is preferable for nitrile introduction) and four-membered carbocycles. The ring contraction to the latter can be done in two ways, in order of
conveniance:
(1) Lead(IV) acetate method. 1,2-dihydro-cyclobutabenzene-1-carboxylic acid has thus been made directly from 1-indanone in 75 %
yield.[5]
(2) α-oximation of the indanone, α-diazo ketone and photo-Wolff rearrangement of it to carboxylic acid.[6]
Resulting carboxylic acids can in turn can be amidated and reduced with NaBH4/I2.
| Quote: | | Also, if you obtain the desired intermediate, how easy (how many reaction) will it be to have the aromaticity of the new benzoquinone derivative
restored? It's not as simple as the reduction of benzoquinone to hydroquinone (as far as my knowledge reaches). |
It's highly likely that α,β-unsaturation of the formed intermediate into benzoquinone can be done in one step under very mild conditions,
but I'm unable to refer to that paper for a personal reason.
References:
[1]: http://www.orgsyn.org/orgsyn/prep.asp?prep=cv7p0315
[2]:
Photochemical synthesis at low temperatures. III. Ready synthesis of bicyclo[4.2.0]octan-2-ones from cyclohexenones and ethylene
Owsley, Dennis C.; Bloomfield, Jordan J. Journal of the Chemical Society [Section] C: Organic (1971), (20), 3445-7.
Abstract: Photochem. [2+2] cycloaddns. of CH2:CH2 to 2-cyclohexen-1-one and 3,5,5-trimethyl-2-cyclohexen-1-one at -70° in CH2Cl2
gave 90% bicyclo[4.2.0]octan-2-one and 85% 4,4,6-trimethylbicyclo[4.2.0]octan-2-one, resp. Under the same conditions, or in Me2CO at -70°,
H-C-tripplebond-C-H did not undergo a [2+2] cycloaddn.
[3]: http://www.orgsyn.org/orgsyn/prep.asp?prep=cv6p1024
[4]:
An efficient and remarkably regioselective synthesis of benzocyclobutenones from benzynes and 1,1-dimethoxyethylene
Robert V. Stevens, Gregory S. Bisacchi.;
J. Org. Chem.; 1982; 47(12); 2393-2396.
Link: http://rapidshare.com/files/170498/Aryne_benzoycyclobutenone...
[5]: Nongrum, F.M.; Myrboh, B.; Synthesis; 9; 1987; 845-846.
Link: http://rapidshare.com/files/172865/s-1987-28099.pdf.html
[6]: http://www.orgsyn.org/orgsyn/prep.asp?prep=cv6p0840
[Edited on 22-10-2006 by Sandmeyer]
|
|
|
Sergei_Eisenstein
Hazard to Others
  
Posts: 290
Registered: 13-12-2004
Location: Waziristan
Member Is Offline
Mood: training
|
|
Another question that comes up to mind concerns the stability of cyclobutabenzene derivatives (I have no practical experience with this type of
substances). They are also used as precursors for o-quinodimethanes, and as such are a valuable type of compounds for pericyclic reactions. To do so,
the cyclobutabenzene is subjected to an electrocyclic ring opening. Some substances require heating, others nothing more then room temperature. The
main problem here is the stability of the o-quinodimethane: it is highly reactive. If there are no reaction partners, there will simply be an
equilibrium between the cyclobutabenzene and the o-quinodimethane, but if there are, I suspect it will become a mess. The o-quinodimethane will
happily react Diels-Alder style. Among others, I see a benzoquinone offering itself for reaction.
I haven't read the complete article yet, but perhaps you can also ask yourself whether the substance analyzed after synthesis, the compound put on the
receptors, and the dust that one day will inevitably will be subjected to gastric inspection by the hippies are the same compound.
Perhaps you should just try, and if it doesn't work out as you hoped, just come back and read what might have gone wrong 
damnant quod non intelligunt
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Sandmeyer 1-Aminomethylbenzocycloalkanes: Conformationally Restricted Hallucinogenic Phenethylamine Analogues as Functionally
Selective 5-HT2A Receptor Agonists1 McLean, T. H.; Parrish, J. C.; Braden, M. R.; Marona-Lewicka, D.; Gallardo-Godoy, A.; Nichols, D. E. J. Med.
Chem.; (Article); 2006; 49 (19); 5794-5803. DOI: 10.1021/jm060656o
http://rapidshare.com/files/79842/New_Nichols.pdf.html
The route to the compound that is of LSD's potency seems rather tedious. To get the key precursor, I was having in mind that one can take
allylbromide, react with potassium phthalimide to get the masked allylamine and let this undergo a [2+2] photoaddition with benzoquinone under
standard conditions, for conveniance... Ideas?
|
This file has been deleted due to alleged inactivity; someone please re-upload it.
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Reference Information
1-Aminomethylbenzocycloalkanes: Conformationally Restricted Hallucinogenic Phenethylamine Analogues as Functionally Selective 5-HT2A Receptor
Agonists1
McLean, T. H.; Parrish, J. C.; Braden, M. R.; Marona-Lewicka, D.; Gallardo-Godoy, A.; Nichols, D. E.
J. Med. Chem.; (Article); 2006; 49(19); 5794-5803.
Abstract
A series of conformationally restricted analogues of the hallucinogenic phenethylamine 1 (2,5-dimethoxy- 4-bromophenethylamine, 2C-B) was synthesized
to test several hypotheses concerning the bioactive conformation of phenethylamine ligands upon binding to the 5-HT2A receptor. These benzocycloalkane
analogues were assayed for their receptor binding affinity and ability to activate downstream signaling pathways, and one exceptional compound was
selected for testing in an in vivo drug discrimination model of hallucinogenesis. All compounds were examined in silico by virtual docking into a
homology model of the 5-HT2A receptor. On the basis of these docking experiments, it was predicted that the R enantiomer of benzocyclobutene analogue
2 would be the most potent. Subsequent chemical resolution and X-ray crystallography confirmed this prediction, as (R)-2 proved to be equipotent to
LSD in rats trained to
discriminate LSD from saline. Thus, we propose that the conformation of 2 mimics the active binding conformation of the more flexible phenethylamine
type hallucinogens. In addition, (R)-2 is one of the most potent and selective compounds yet discovered in the in vivo drug discrimination assay.
Further, 2 was found to be a functionally selective agonist at the 5-HT2A receptor, having 65-fold greater potency in stimulating phosphoinositide
turnover than in producing arachidonic acid release. If hallucinogenic effects are correlated with arachidonic acid production, such functionally
selective 5-HT2A receptor agonists may lack the intoxicating properties of hallucinogens such as LSD.
Attachment: New_Nichols.pdf (194kB)
This file has been downloaded 1755 times
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
Sergei_Eisenstein
Hazard to Others
  
Posts: 290
Registered: 13-12-2004
Location: Waziristan
Member Is Offline
Mood: training
|
|
| Quote: | | If there are no reaction partners, there will simply be an equilibrium between the cyclobutabenzene and the o-quinodimethane, but if there are, I
suspect it will become a mess. The o-quinodimethane will happily react Diels-Alder style. Among others, I see a benzoquinone offering itself for
reaction. |
I was wrong about benzoquinone being a possible reaction partner, since I assume you do the aromatization in at least a second step. Nevertheless, the
o-quinodimethane intermediate remains a theoretical possibility.
damnant quod non intelligunt
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
I come to like this idea of photocycloaddition (BTW, great idea, Sendmayer!). However I don't have Beilstein/SciFinder access during the weekend. The
only references regarding [2+2] photoadditions on p-benzoquinone I was able to find using keywords are all at the RSC publisher that is currently
offline due to maintenance.
The HOMO/LUMO energy difference is satisfactory even though the N-allyl-phthalimide is not electron rich, but the Paterno-Büchi side reaction is
still possible (I think saw preparative examples on p-benzoquinone as well, but more on this when I will be able to get the papers).
As Sergei noted there might be a problem with the stability of the resulting cyclobutabenzene. Would they survive methylation and deprotection with
hydrazine? From Nichols we know they survive BH3 and Br2, so they are not that very sensitive, but here we would have a phenolic form first.
Synthesizing an analogous 5-HT2A agonist from p-naphtaquoinone would allow for skipping the bromination and the synthesis would require only 3 steps
(with the possibility that the photoaddition works better on naphtoquinones?).
| Quote: | Originally posted by Sandmeyer
It's highly likely that α,β-unsaturation of the formed intermediate into benzoquinone can be done in one step under very mild conditions...
|
I think you and Sergei both neglected that the resulting intermediate of the [2+2] photocycloaddition tautomerizes to the already aromatic compound
(that's the beauty of working with quinones – see scheme). So there is no need to do anything else to it than simply methylate with
dimethylsulphate.
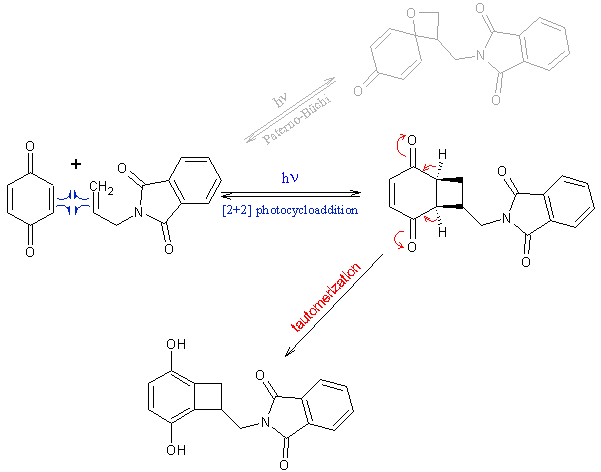
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
caribou
Harmless

Posts: 29
Registered: 21-2-2006
Member Is Offline
Mood: No Mood
|
|
There is at least one more route to 1-(aminomethyl)benzocyclobutenes, i. e. by intramolecular nucleophilic substitution in
3-(2-bromophenyl)propanenitrile anions (generated from the nitriles by deprotonation with LDA or sodium amide).
EP43194.
The method was applied in the synthesis of methoxy-substituted 1-(aminomethyl)benzocyclobutenes which were O-demethylated and evaluated as
adrenergic agonists.
The same authors obtained 1-(aminomethyl)indanes, tetralines and phthalans for the same purpose.
J. Med. Chem. 1985, 28, 1398;
J. Med. Chem. 1986, 29, 463;
J. Med. Chem. 1987, 30, 178.
1-(Aminomethyl)-5,6-dimethoxyindane is a known compound. It was obtained from 5,6-dimethoxy-1-indanone by 2 methods:
1) a) TosMIC/t-BuOK; b) LAH and
2) a)BrCH2CO2Et/Zn; b) H2 - Pd/C; c) N2H4; d) HNO2; Curtius rearrangement; hydrolysis.
Org. Prep. Proc. Int. 1992, 24, 45;
Bol. Soc. Chil. Quím. 2002, 47(1), 25-31.
The articles mentioned are in the attached archive.
Attachment: constrained_PEAs.zip (402kB)
This file has been downloaded 660 times
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
Hi Caribou. I didn't see the advantage over Nichol's route since one has to make these propanenitriles, quite a formidable task.
Anyways, someone please get this paper:
Oda, Masaji; Kanao, Yoshinori; Chem. Lett.; 1981; 37-38.
bicyclo[4.2.0]oct-3-ene-2,5-dione (the cycloadduct between benzoquinone and ethylene) --THF/RoomTemp--> 1,2-dihydrocyclobutabenzene-3,6-diol
(beloved hydroquinone) Yield: 84%
In SMILE code:
O=C(C=C1)C2C(CC2)C1=O ----> OC1=C2C(CC2)=C(O)C=C1
[Edited on 22-10-2006 by Sandmeyer]
|
|
|
caribou
Harmless

Posts: 29
Registered: 21-2-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | | Oda, Masaji; Kanao, Yoshinori; Chem. Lett.; 1981; 37-38. |
It must be freely accessible:
PDF, 211 KBytes, 2 pages.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
That Chem. Lett. paper is really cool, though it surprises me that the tautomerization to the aromatic form needs to be catalyzed by DBU. I was
convinced it occurs spontaneously in the slightest presence of any acid or base (I was wrong). Someone with full Tetrahedron letters access please
upload the reference 4.
Caribou, thanks for the interesting papers. That nitrile ring closure is a viable option, but I have to agree with Sandmeyer on this - it is too much
work. Nevertheless, here is another paper relevant for such a route, probably the oldest one:
J. F. Bunnett and Joseph A. Skorcz. Homocyclic Ring Closures via Benzyne Intermediates. A New Synthesis of 1-Substituted Benzocyclobutenes.
Journal of Organic Chemistry, 1962, 27(11), 3836 – 3843.
Attachment: Homocyclic Ring Closures via Benzyne Intermediates.pdf (916kB)
This file has been downloaded 888 times
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Ullmann
Hazard to Self
 
Posts: 51
Registered: 22-12-2004
Member Is Offline
Mood: No Mood
|
|
What is the problem with the same route than the authors but generating the aryne from bromo-dimethoxybenzene and NaNH2 or LDA? It is much easier to
get this precursor than the anthranilic acid they use. Their route is viable and short only three steps from 1,4-dimethoxybenzene and they are easy
steps, if you can get the acrylonitrile and strong base this is the best way to do it IMHO.
Anyway altough i didnt found a ref with generation of a beta-amino cyclobutane compound from benzoquinone i have found a ref about the alcohol
analogue protected by an acetate group, starting from allyl-acetate.
It is online from the same site than above, check : chem. Lett. 1986 (12) 2113-16
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
That paper (from the same authors) does not use p-benzoquinone but 5,6-dichloro-2-cyclohexene-1,4-dione. Nevertheless, this would be just perfect if
there is some simple way to prepare this dichloro, or preferentially dibromo, compound (Could it be as simple as a halogenation of p-benzoquinone
under conditions where no HX elimination or tautomerization occurs? These stupid Letters, they never have the needed procedures!).
Unfortunately Nichols' method limits the formation of the benzyne intermediate to neutral media since there is no way to prevent acrylonitrile
degradation/polymerisation in NaNH2/NH3. As for using the benzyne method with substrates such as N-allyl-phthalimide or N,N-dibenzyl-allylamine, I'm
not sure whether it would work. I'm not familiar with benzyne chemistry involving [2+2] cycloaddtions. Anyway, here is paper on the usage of
2-bromo-1,4-dimethoxybenzene as a benzyne source for further reactions (in case someone finds it inspiring for anything else but a banal
2-(2,5-dimethoxyphenyl)acetonitrile synthesis):
Yu Xin Han, Misa V. Jovanovic, Edward R. Biehl. Reaction of 2-bromo-1,4-dimethoxybenzene with various nucleophiles via aryne reaction. J. Org.
Chem., 1985, 50(8), 1334-1337.
[Edited on 22-10-2006 by Nicodem]
Attachment: Reaction of 2-Bromo-1,4-dimethoxybenzene with Various Nucleophiles via Aryne Reaction.pdf (414kB)
This file has been downloaded 931 times
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Reference Information Requested
Preferential photochemical cyclobutane-ring formations of 2-cyclohexene-1,4-dione with olefins and acetylenes. A simple and general synthesis
of bicyclo[4.2.0]octane-2,5-diones
Masaji Oda, , Hidetoshi Oikawa, Yoshinori Kanao and Akira Yamamuro
Tetrahedron Letters, Volume 19, Issue 49, 1978, Pages 4905-4908
Attachment: Preferential Photochemical cyclobutane ring formation.pdf (258kB)
This file has been downloaded 969 times
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
Check out this wonderful paper, from the same japanese group:
http://www.journalarchive.jst.go.jp/jnlpdf.php?cdjournal=cl1...
It would be fucking fantastic if one could go from trans-dibromo derivative with the same results.
Ullman, I pointed out refs for that aryne route couple of posts ago, but I doubt one could use acrylonitrile under conditions required to generate
aryne from bromoaryl.
Edit: I missed some of the above, sorry for redundance 
[Edited on 22-10-2006 by Sandmeyer]
|
|
|
Sergei_Eisenstein
Hazard to Others
  
Posts: 290
Registered: 13-12-2004
Location: Waziristan
Member Is Offline
Mood: training
|
|
| Quote: | | It would be fucking fantastic if one could go from trans-dibromo derivative with the same results. |
Reductive dehalogenations are widely described in the literature. If their procedure doesn't work, you may easily find a lot of alternatives.
damnant quod non intelligunt
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Seems like the photocycloaddition does not work on p-benzoquinone or else the Japonese authors would have referred to it. So we are left to find a
simple method for the synthesis of 5,6-dichloro- and 5,6-dibromo-2-cyclohexene-1,4-dione.
The synthesis of 2-cyclohexene-1,4-dione as published in Organic Syntheses is not attractive at all!
(Another relevant OrgSyn link is: tert-Butylcyanoketene – I wander if the first step could be used on plain p-benzoquinone? That would be a winner!)
There can exist the possibility that p-naphtoquinone is on the edge of applicability in this photochemical method, so it would be worth to consider
this option when doing literature research.
| Quote: | Originally posted by Sandmeyer
Anyhow, another option is to go via aryne corresponding to 2-bromo-p-dimethoxybenzene. Once generated, this species
a.) may be able to undergo a [2+2] with, N,N-dibenzylallylamine* or N-allylphtalimide** to give protected amine directly
[....]
Ullman, I pointed out refs for that aryne route couple of posts ago, but I doubt one could use acrylonitrile under conditions required to generate
aryne from bromoaryl.
|
Someone here around has a batch of 2-bromo-1,4-dimethoxybenzene to play with, so let's also research this route. In particularly, I would like to see
references of [2+2] cycloadditions on benzyne formed from bromobenzenes. More particularly, on N-allyl-phthalimide or equivalents.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Ullmann
Hazard to Self
 
Posts: 51
Registered: 22-12-2004
Member Is Offline
Mood: No Mood
|
|
The dibromo-quinone is easily made by addition of bromine to a quinone solution in DCM at 0°C in a yield of ~70%. I think you have to start either
from this enedione or the non-halogenated one you posted Nicodem otherwise there is a risk to form a bis-substituted diketone ie a bis-cyclobutane at
both side of the benzoquinone.
You do not have to generate the aryne in liquid ammonia, solid NaNH2 in THF or even KOtBu IIRC are regularly used for such arynic formation from
variously substituted halobenzenes. I do not think that the acrylonitrile is that unstable in such conditions, it may be possible. On the other hand,
the only references i saw about 2+2 benzyne-alkene cycloaddition all involved preparation of the reactive aryne from the zwiterrionic diazonium salt.
Another problem may be quenching of the aryne with the reactant ie the base to make the aniline or O-tert-butylphenol counterpart, hence i think best
experimental protocol would involve slow addition of the base to a solution of the halo-benzene in excess acrylonitrile. I do not now the relative
rate of the quenching nucleophilic reaction vs the 2+2 cycloaddition. At least with acetonitrile anion this species was more reactive than the
NaNH2/NH3 they used but i cannot affirm than the rate of cycloaddition will be sufficient in our case. Better use an hindered base like LDA or KOtBu
as a base IMHO...
Problem with the paper i gave the link is that you have a mixture of regioisomeric halodimethoxybenzenecyclobutanes at the end, which means
chromatography separation of the products probably. Anyway it looks doable.
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
| Quote: | Originally posted by Ullmann
Problem with the paper i gave the link is that you have a mixture of regioisomeric halodimethoxybenzenecyclobutanes at the end, which means
chromatography separation of the products probably. Anyway it looks doable. |
Nicols obtains a 5:1 excess of para regioisomer on bromination of "cyclobutane-2-CH". The japanese paper gives 3:1 ratio to our favour. Going from
trans-dibromo derivative, the isomers could be separated at the final stage by simple recrystallisation, just like described by Nichols...
|
|
|
Drunkguy
Hazard to Others
  
Posts: 172
Registered: 23-12-2005
Member Is Offline
Mood: somewhat pissed.
|
|
This is kinda related to the Diels Alder reaction? I have experience doing those. [I have an "Oxford Primer" book on pericyclic reactions but I dont
use it at all. I will happily scan it if anyone wants an e-copy].
4 + 2 cycloadditions are very common. However these 2 + 2 stuff is alot more exotic and I dont know about it OTTOMH.
You can make the precursor between phthalimide and allyl bromide, right?
I read a little about 2 + 2 cycloadditions last night, you are absolutely right that the 4n + 2 rule means that UV light is necessary. However it also
appears that this reaction must be done at sub-zero temperatures.
In the reaction mechanism you showed 4 curly arrows. Just wondering if hv mediated process is free radical or if that was a typo.
[Edited on 23-10-2006 by Drunkguy]
[Edited on 24-10-2006 by Drunkguy]
[Edited on 24-10-2006 by Drunkguy]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
More literature for more hope...
I found more papers from Iyoda et al on the phtocycloadditions on 5,6-dichloro-cyclohex-2-ene-1,4-dione. In the Synthesis (1986, 322-324) paper
(attached) they used many simple non-conjugated alkenes. There is thus little doubt that N-allyl-phthalimide would work as well.
As for the synthesis of 5,6-dichloro-cyclohex-2-ene-1,4-dione and 5,6-dibromo-cyclohex-2-ene-1,4-dione, it is just as simple as Ullman said. Iyoda et
al used the method from Can. J. Chem., 1966, 44, 2869-2872. Other synthesis references follow:
5,6-dichloro-cyclohex-2-ene-1,4-dione from p-benzoquinone:
Tetrahedron, 2003, 59(8), 1349 - 1358.(SO2Cl2/Et3N/Et2O)
Recl. Trav. Chim. Pays-Bas, 1954, 73, 5. (Cl2/CHCl3)
J. Chem. Soc. Perkin Trans. 1, 1981, 2670-2676. (Cl2/CHCl3)
5,6-dichloro-cyclohex-2-ene-1,4-dione directly from hydroquinone:
Gazz. Chim. Ital., 1894, 24 II, 384. (SO2Cl2/Et2O)
5,6-dibromo-cyclohex-2-ene-1,4-dione from p-benzoquinone:
Zh. Obshch. Khim., 1960, 30, 2316. = J.Gen.Chem.USSR (Engl.Transl.), 1960, 30, 2296. (Br2/HCl(aq))
Dokl. Akad. Nauk SSSR, 1935, II 244-248. (Br2/HCl(aq))
Chem. Zentralbl., 1936, 107, I 2537. (Br2/HCl(aq))
Tetrahedron, 1994, 50(12), 3709-3720. (Br2/CH2Cl2, obtained in mixture with 2,5-dibromo-p-benzoquinone)
Chem. Europ. J., 2001, 7(8), 1619 - 1629. (Br2/CH2Cl2)
The resume of the general idea of what is expected to work based on analogous literature examples (modifications leading to the bromo version are
applicable):
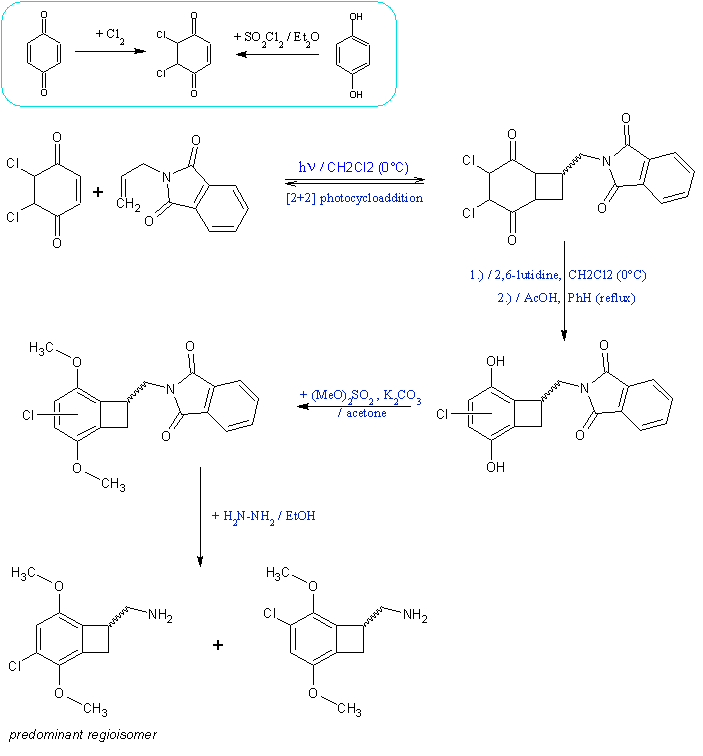
The idea of using ketene dimethyl acetal cycloaddition on arynes formed from bromoaromates, already described by Sandmeyer, was actually tried on
2-Bromo-1,4-dimethoxybenzene as well:
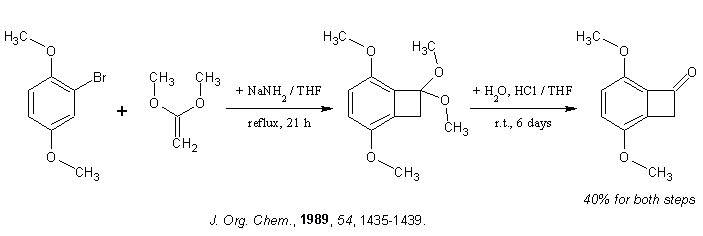
3,6-Dimethoxybenzocyclobutenone (5e). 1-Bromo-2,5-dimethoxybenzene (10 g, 0.046 mol), 8.1 g (0.092 mol) of ketene dimethyl
acetal, and 3.6 g (0.092 mol) of sodium amide were refluxed in 20 ml of THF for 21 h. The crude ketal was isolated and hydrolyzed for 6 days at room
temperature in 100 ml of 10% HCl with 20 ml of THF cosolvent. Chromatography on a 100 x 5 cm silica gel column with 25% ethyl acetate in hexanes and
then recrystallization from hexanes gave 3.3 g (40%) of yellow product. Mp: 104°C (hexanes) ... + IR & NMR data...
Taken from (attached):
Lanny S. Liebeskind, Leonard J. Lescosky, and Charles M. McSwain, Jr. Synthesis of Substituted Benzocyclobutenediones J. Org. Chem.,
1989, 54, 1435-1439.
Whether or not acrylonitrile is applicable and if it can be used in the presence of NaNH2 is still a mater of literature research...
Attachment: benzocyclobutanes.zip (862kB)
This file has been downloaded 738 times
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Drunkguy
This is kinda related to the Diels Alder reaction? I have experience doing those. [I have an "Oxford Primer" book on pericyclic reactions but I dont
use it at all. I will happily scan it if anyone wants an e-copy]. |
It bears some similarities with the Diels-Alder reaction, but it is mechanistically quite different. Diels-Alder reactions are [4+2] cycloadditions,
initiated thermally while photocycloadditions are [2+2] and are not speeded up thermally. However the main difference is in the HOMO/LUMO interaction
which is completely different. The Guidebook to Mechanism in Organic Chemistry (Sykes, 1986), (available as e-book) gives the very basics of the
mechanisms of all pericyclic reactions. However, for more than just basics, you would need other sources – I don't know what is the book you have
but scanned books are always welcomed here. 
| Quote: | | You can make the precursor between phthalimide and allyl bromide, right? |
Yes. The preparation of N-allyl-phthalimide is trivial, hence the proposal of its use. It can also be prepared from allylamine and phthalanhydride.
| Quote: | | I read a little about 2 + 2 cycloadditions last night, you are absolutely right that the 4n + 2 rule means that UV light is necessary. However it also
appears that this reaction must be done at sub-zero temperatures. |
It is true that often they are being performed in dry ice/acetone bath temperature, but not always. For example, this particular
5,6-dichloro-cyclohexen-1,4-dion substrate is irradiated at 0°C. The cooling is needed also because of the heat caused by light absorption, thus to
prevent any thermal side reaction.
| Quote: | | In the reaction mechanism you showed 4 curly arrows. Just wondering if hv mediated process is free radical or if that was a typo.
|
No, I don't think you can consider them as free radical processes. The reason I used double half-arrows is because photochemical reactions of Pi-bonds
are generally considered as biradical interactions, though in reality this is probably another simplified view. Besides, I still have to learn more
about photochemistry in general as I know not much more than what was thought in the school.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Drunkguy
Hazard to Others
  
Posts: 172
Registered: 23-12-2005
Member Is Offline
Mood: somewhat pissed.
|
|
Download link here:
http://www14.rapidupload.com/d.php?file=dl&filepath=6146
I have quite alot of these Oxford Primer books btw.
|
|
|
| Pages:
1
2 |
|