RogueRose
International Hazard
    
Posts: 1594
Registered: 16-6-2014
Member Is Offline
|
|
Maintaining heat level of filtrate before getting to filter - various methods possible
Sometimes i need to filter a solutions which is best filtered at as high a temp as possible (as close to 212 as possible), usually for solubility or
viscosity issues. I'm wondering what ways people use to keep the temp at a desired level right before or while in the filter.
When filtering something that has a difference of like 3kg/L & 100C vs 1.2KG at 40C, this makes a really big difference, or if filtering oil, it
gets difficult when it thickens up. For the most part I use an inline canister type filter with a hose barb on either end.
I was thinking of using vinyl tubing run through a length of black garden hose and having the hose on a black driveway or some other surface that will
both attract and retain heat. I would fill the hose with water and allow it to sit in the sun. I have many LARGE Fresnel lenses (1m^2 - 1.7m^) that
could be used somehow (focus on a sealed container/pot with in/out hose connectors , maybe circulate the water in the garden hose and the inner vinyl
tube would be heated by the water in the hose. If it isn't sunny then the container could be put over a flame of some type or on a heating element.
Another idea is using a "reverse worm" that moonshiners use as a condenser. Take 5-25 ft of Cu/Al or SS tubing and put it in a 5 gallon bucket or a
stock pot with hot water (stove, gas grill, immersion heater - lots of ways to heat the stock pot) bur the 5 gallon is a little more difficult to
figure out.
Anyway, if anyone does this, I'd be interested in hearing how you keep the temp high while you do your filtering.
|
|
|
Orenousername
Hazard to Self
 
Posts: 79
Registered: 16-4-2016
Location: USA
Member Is Offline
Mood: Regulated
|
|
How much solution are you trying to filter? Usually I simply pour boiling solutions into my buchner funnel. This works well for small amounts
(~250mL) but it seems you're trying to process large amounts. I'd suggest just insulating and filtering smaller amounts at a time, keeping the
solution hot (on a hotplate/stove etc).
Lol nerds
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
An suitably sized electric immersion heater would be ideal for your purposes.
You could also filter it batchwise, pouring a liter or so at a time with reheating in between.
For hot filtering I usually keep my buchner funnels in boiling water or an oven (depending on water sensitivity of filtration) so solutions filter a
lot more quickly. Otherwise, the cool frit causes things to crash out of the solution and clog it.
|
|
|
Dr.Bob
International Hazard
    
Posts: 2736
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
There are jacketed Buchner funnels that you can cool or warm with water, steam, or chilled glycol. You can find similar items in glass frits as
well, but most are 150 mm or less. Once you get above that, most people go to fixed reactors and just drain the bottom through a filter unit which
has a low hold up volume and can be insulated or jacketed. Simplest solution is to insulate the funnel, as if it starts hot, it will stay that way
if well insulated.
|
|
|
CaptainPike
Hazard to Self
 
Posts: 68
Registered: 21-12-2012
Member Is Offline
Mood: No Mood
|
|
Hot Filtration
I'm getting better at using a technique I read about somewhere (great reference, huh). And this may be a little off-topic, since I'm not sure what is
being filtered. But this simple method works great for somewhat lower melting point compounds being recrystallized.
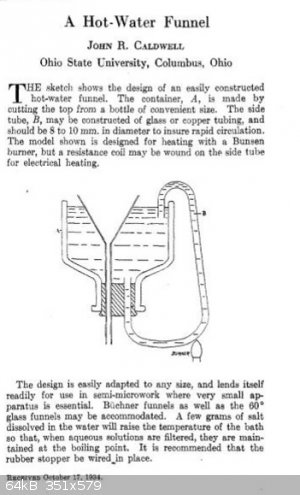
In Vogel it talks about using a "hot water funnel", for the recrystallization of beta nitro styrenes. I don't have one of these, and it seemed like a
goofy kind of thing to create. So this other method involves putting a little bit of the recrystallization solvent in where the filtrate will wind up,
and heat this, along with a beaker (or whatever) of the rest of the recrystallization solvent.
I'm using a small fritted, vacuum filter funnel along with and Erlenmeyer flask for the filtrate (where I have a few milliliters of pet ether). This
might be better than ethanol because it can get hotter, I think the nitrostyrene melts in the middle 50s, so this helps to keep it solvated(it may
also reduce yield by decomposing product). The point being you don't want your filtrate to crystallize in the funnel and block the area below the
frit, or the tube, as it cools.
Vacuum filtration of a hot, flammable, low boiling solvent could be thought of as a crazy idea, unless it's the best way to go and you know what
you're doing.
But the little bit of solvent, almost boiling in the flask helps to heat up the filtering glassware, thus preventing early crystallization.
My first time around I used ethanol. I also used my little KNF vacuum pump to filter the crude nitrostyrenes. The filter got blocked and I'm pulling a
vacuum on hot ethanol. This flooded of my diaphragm pump, and started spraying hot solvent containing my solidifying product into the air (and onto
the hotplate). I know I know, I should have a trap. I do have a trap – I must unpack it. We all learned what"lachrymatory" meant that day, I hate to
admit! So for once, I would've preferred a lower vacuum, or just using an aspirator for the filtration.
|
|
|