| Pages:
1
2 |
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Preparation of Sodium Bisulphite n stuff
Researching what was needed to try to replicate Magpie's Congo Red synthesis (http://www.sciencemadness.org/talk/viewthread.php?tid=66391#...) and reading the Kline document, it became apparent that i was lacking seven
reagents, namely :-
sodium carbonate
sodium bisulphite
conc hydrochloric acid
conc nitric acid
sodium nitrite
benzene
anhydrous magnesium sulphate
Puts me in mind of one of zts16's posts where he said he had a Target and was working towards it - a very good idea IMHO.
First, and easiest : anhydrous magnesium sulphate
MgSO4 in oven. Turn on, highest setting, forget.
Next easiest : sodium carbonate
OTC Bicarbonate of Soda (baking soda) decomposes with heating to become sodium carbonate, water and carbon dioxide.
2 NaHCO3 heat=> Na2CO3 + H2O + CO2
The stoichimetry determines an End Point when the starting material loses roughly 37% of it's weight (as steam and carbon dioxide).
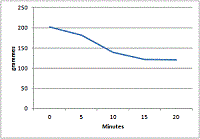
200g was put in a saucepan and heated on a gas hob, then weighed every 5 minutes. After 15 minutes the pre-calculated end-weigh was reached. It was
left on the heat for another 5 minutes although no further weight loss was observed.
What's left in the pan is sodium carbonate.
Surprisingly the curve from the weight data was not a straight line :
Next up: sodium bisulphite
Ref: http://www.prepchem.com/synthesis-of-sodium-bisulfite/
Basically gas a saturated solution of sodium carbonate with sulphur dioxide.
Na2CO3 + 2 SO2 + H2O => 2 NaHSO3 + CO2
36g of 15mm copper pipe was cut into rings using a pipe cutter and loaded into a 250ml flat-bottomed flask (FBF) on a hotplate. Stirring was not
required.
250ml of conc H2SO4 was put in a pressure equalising addition funnel (PEAF) above the FBF containing the copper.
A gas take-off adpater was fitted to the top of the PEAF and a plastic tube led the gas to the bottom of a 250ml 3-necked RBF, which was loaded with
distilled water.
These three elements form the SO2 generator. (i presume that the water wash removes SO3, which ignites some ideas of making
oleum ...)
One neck was stoppered and the remaining neck led the gas to the bottom of a 500ml RBF which was loaded with a saturated solution of
Na2CO3 (100g in ~300ml water) i.e. the reaction vessel.
A vac adapter was used to allow the reaction vessel to vent gas into a standard inverted funnel NaOH trap.

Heating was started and the sulphuric acid added. The addition funnel serves as an anti-suck-back device when the valve is closed.
When SO2 production is in progress, each bubble entering the reaction vessel caused a 'shower' of what is probably the product, mostly when
the bubble pops on the surface.

122 minutes into the reaction there were some flakes forming

After 155 minutes the reaction was sluggish and there were sheets of crystals in the reaction pot.
An attempt was made to vacuum distill off some water, although as-per the procedure, this was uneccessary.
It also bumped way to much so it just got put in the fridge instead.
After 1 hour there were a great many crystals, which were vac filtered out, then washed as per the procedure (cold water then cold ethanol).
Unfortunately no vacuum dessicator is available, so they are now in a ziplok bag with CaCl2, which i think might be a Bad idea, seeing as
it tends to oxidize in air ...
The supernantant is still in the fridge, so either the bag works or i'll try drying again with something - not sure what yet.
I wonder what the by-product of boiling Cu in conc H2SO4 might be ?

Sure looks kinda familiar 
[Edited on 20-6-2016 by aga]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Key question : why does the referenced process for sodium bisulphite specify vac dessication ?
Answer : Oxygen.
The wet product will get oxidised in Air.
They had a fully equipped lab.
Bung it in the vac dessicator.
Job done.
Making a vacuum dessicator seems do-able with a pyrex bowl, a sheet of nylon or similar, a threaded spiggot and some silicon sealant (at least in
theory).
Seems a lot of effort to dry this one reagent, and i'm not happy leaving anything running overnight in the shed apart from the fridge.
Solution: iron oxidises in moist air, and the addition of sodium chloride acts as a catalyst to allow it to work as an oxygen scavenger in very low
moisture-containing air (this was found on wiki by searching 'oxygen scavenger').
BUT it'll need to be very finely divided Iron.
So, make ferrous chloride with some bits of iron (nails would do) and HCl.
Filter the resulting green mess directly into some 3% H2O2 to get yellow/orange ferric chloride, then add (cautiously !) a bar
of aluminium so the Al displaces the Fe to make AlCl3 giving very fine elemental Fe dust.
The addition of Al does nothing for a few seconds, then goes crazy in a quite exothermic reaction, so it was thought best to keep dipping the bar in
and out of the FeCl3 solution, rather than just dunk it in there and run away.
Eventually the chaos subsides and the ferric chloride solution substantially loses it's characteristic colour.
Left to cool overnight, the beaker should have a crop of elemental Fe to decant/filter off, dry, mix with table salt, then add to the ziplock bag to
scavenge out oxygen.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Thanks for posting this synthesis of SO2. I haven't needed one yet but might in the future.
Sodium bisulfite and sodium metabisulfite are so readily available and cheap at the brewing shops that I buy them there. But that's a personal choice
and I always encourage syntheses in general.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Beer is so cheap here that home brewing is basically not cost-effective, hence no shops dedicated to brewing.
Every September a few 'campesinos' collect their moscatel grapes, dry about 20% of them in the sun for a few days, then crush the lot and put the
juice into a big wooden barrel.
40 days later everyone seems very happy, although 'secondary effects' are observable.
Quite a lot of work to get all of the reagents required before even starting an attempt at your Congo Red synth, but that's a few more ticked off the
list : also educational and fun.
Next up is sodium nitrite.
Wonder how hot the lead has to get to oxidise ?
Guess i'll find out.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
You need "a lot" of heat. See the thread on "ionic nitrites."
I would make sure that your nitrite is of good quality. If you fail at this point (in the Congo Red synthesis) you will have wasted a lot of time and
effort.
Again, I buy my NaNO2 now. If you are going to make it verify your quality (somehow).
[Edited on 22-6-2016 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Effort is never wasted when you enjoy the process and learn something from it.
If i fail to make congo red the first time round, well, probably more interesting than if i succeed !
Must have been very exciting times for the early chemists who had to make everything from scratch.
Every single step, even a 'simple' recystallisation, would have to be performed perfectly.
(sodium nitrite is NaNO2, with nitrate being NaNO3)
As per the procedure i found, seems that i need to make at least 40g more sodium nitrate too.
[Edited on 21-6-2016 by aga]
|
|
|
ParadoxChem126
Hazard to Others
  
Posts: 104
Registered: 5-4-2013
Location: USA
Member Is Offline
Mood: No Mood
|
|
Very interesting series of posts, aga!
It is nice to see the preparations of these chemicals which many of us take for granted.
If you do have a vacuum pump, you may be able to dry small amounts by pulling a vacuum on an RBF containing the compound while providing radiant heat
from a tungsten bulb.
Keep up the nice work!
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Thanks. It is quite fun to make all these things.
Having read the second, longer thread, i now have a test for nitrite, which is great - thanks.
http://www.sciencemadness.org/talk/viewthread.php?tid=52
"one drop of 6N (20%) HCl on it. There was an immediate and strong reaction giving a brown gas" 
The Pb method does sound hit-and-miss, although i should give it a go.
|
|
|
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
Nice thread! It's nice to see people doing chemistry once in a while. Do let us know whether the Fe/NaCl mixture works! (will you grind the NaCl more
finely?)
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
The FeCl3 route to finely divided iron didn't work.
Bits of Al get everywhere making it impossible to tell what the black crud is.
ParadoxChem126's idea sounds more likely to work.
Whilst messing about with that, the sodium bisulphite seems to have dried enough to go into a bottle.
[Edited on 23-6-2016 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  |
The FeCl3 route to finely divided iron didn't work.
Bits of Al get everywhere making it impossible to tell what the black crud is.
|
Any finely divided Fe formed that way would rust like mad anyway. Fe is just too electropositive to be prepared that way.
Buying fine Fe powder should not be a big problem though.
Nice work on the bisulphite!
[Edited on 23-6-2016 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
There was not enough NaNO3 to attempt making sodium nitrite by any method, so more sodium nitrate had to be made.
Having on hand a large qty of agricultural 56% HNO3 made the direct route simplest in my situation.
Sodium Nitrate synthesis
Sodium salt + Nitric acid => Sodium Nitrate.
You can use sodium bicarbonate, sodium carbonate or sodium hydroxide etc, add it to nitric acid. I used sodium carbonate.
Na2CO3 + 2HNO3 => 2NaNO3 + CO2 + H2O
50g of NaNO3 will make a max of 80g NaNO3.
Based on this we need 59.5g of nitric acid.
As it is 56% nitric acid, we need around 107g.
The acid was added to a plastic 250ml drinks cup and the sodium carbonate powder added by spatula, waiting for the fizzing to stop between additions.
Some heat was generated, although not enough to reach boiling point.
(if NaOH were used the reaction would be MUCH MORE exothermic !).
After addition of all the sodium carbonate the mixture had 5ml more HNO3 added to ensure some acidity, was stirred for 5 minutes then
filtered.
Solubility tables dictate that our 80g of product will dissolve in 44ml of water at 100 C, and only half will dissolve in this volume at 25 C, so the
resultant 170ml of liquid was transferred to a beaker and boiled down to roughly 50ml.
On cooling to RT a large crop of crystals precipitated and the supernantant was poured off into a smaller beaker.

Wiki says that NaNO3 is insoluble in acetone, so approx 50ml acetone was added to the supernantant.
After a few seconds the mixture formed three layers : the acetone on top, the NaNO3(aq) below and NaNO3(s) crystals at the
bottom.

At the interface of the two liquid layers was a continuous 'snow' of crystals falling. Amazing to watch.
A strange effect was that if any crystals on the glass in the acetone layer were scratched off with a glass rod, more crystals quickly (40 secs)
formed precisely where the glass rod had touched the glass wall of the beaker.
Once the crystal 'snow' stopped (~15 mins) the acetone + remaining supernatant liquids were decanted off, leaving pristine white crystals.
By comparison, the first crop of crystals still had a slight green colour due to contaminants in the nitric acid :

The crops were combined and treated twice with acetone, both times left to settle then the green-tainted acetone poured off.
An attempt was made to recrystallise the entire mass from ethanol. This failed to dissolve the sodium nitrate very much at all.
The ethanol/acetone wet product weighed 65.59g, representing 82% yield, although after an hour of drying it weighed 56.26g = 70%, and it has not
finished drying yet.
Still, nice to get pure white crystals !

Edit:
After drying overnight/all day today, the final weight is 53.5g = 67% yield.
[Edited on 25-6-2016 by aga]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Trying very hard not to have to boil lead for an hour in order to make sodium nitrite i came across this (dubious) reference : http://sci-toys.com/ingredients/sodium_nitrite.html
The relevant part is "Sodium nitrite is produced in the human body by the action of saliva on sodium nitrate"
I was taught many moons ago that saliva contained alpha-amylase which is for starch breakdown, although it must contain much more.
NaNO3 and dribble are available, so an experiment is in order.
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
On your list is Nitric acid and Sodium Bisulfate...Of course you say you have a large supply of HNO3, but if you ever needed to make it,
you'd get sufficient bisulfate as a byproduct.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
If i used sodium nitrate and boiled that nitrate salt with sulphuric acid to make WFNA, would the boiling pot contain sodium bisulphate ?
I imagined it to be just sodium sulphate, so recrystallised it three times and bottled it.
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Yes (unless you used more than a 1:1 ratio of sodium nitrate to sulfuric acid). I have personally made sodium bisulfate this way.
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Quote: Originally posted by aga  | If i used sodium nitrate and boiled that nitrate salt with sulphuric acid to make WFNA, would the boiling pot contain sodium bisulphate ?
I imagined it to be just sodium sulphate, so recrystallised it three times and bottled it. |
Yep. The Prax/Doug's Lab video about making nitric acid is good, explains that and a few other things. Also, uses a ridiculous apparatus to do
it...
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Cheers TVC. That is indeed a great video.
|
|
|
myristicinaldehyde
Hazard to Others
  
Posts: 166
Registered: 23-4-2016
Location: .͐͌ ͛҉̻̫̰̻̖E̮ͮ̐́̚ ̢̗̅̉ͩ͂̒̌.̯̻̺̯̀̎͂̄ͩ̚
Member Is Offline
Mood: сорок пять
|
|
Aga, when you used copper pipe, a more aggressive way is to use copper powder instead, and then add acid. This can be made from copper chloride and
aluminum foil, the chloride is made in situ by an excess of NaCl in a copper sulfate solution, if need be.
I never set up a SO2 generator, but if you want to use this powder, you must move fast- it oxidized in my air to a green powder (think Statue of
Liberty dandruff) in 4 hours!
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Clearly, even to a dullard like me, powdered copper would react far too quickly.
Please do not send any more U2Us with stupid suggestions for reactions that would explode.
|
|
|
myristicinaldehyde
Hazard to Others
  
Posts: 166
Registered: 23-4-2016
Location: .͐͌ ͛҉̻̫̰̻̖E̮ͮ̐́̚ ̢̗̅̉ͩ͂̒̌.̯̻̺̯̀̎͂̄ͩ̚
Member Is Offline
Mood: сорок пять
|
|
Ah, the luxuries of working with concentrated sulphuric acid! I was not trying to kill you. However, for the chemically deprived (like me) I was under
the impression it could make even shitty acid effective.
Edit: aga, you meant reply, right?
[Edited on 2016-6-29 by myristicinaldehyde]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quote: Originally posted by myristicinaldehyde  | Ah, the luxuries of working with concentrated sulphuric acid! I was not trying to kill you. However, for the chemically deprived (like me) I was under
the impression it could make even shitty acid effective.
Edit: aga, you meant reply, right?
[Edited on 2016-6-29 by myristicinaldehyde] |
Quoted just to keep it here and public.
Definitely time for bed. Night night.
[Edited on 29-6-2016 by aga]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
While waiting for a nitric distillation to finish, tonight's side-project was to purify some mothballs.
Amazing that nobody ever said how easy, and Wonderful recrystallising napthalene is !
Simply crush up your napthalene mothball (one of the original smelly types, not a modern pesiticide-based one), add methanol, heat+stir until it
boils, or you're not happy with how hot it's getting, filter.
A few seconds/minutes later a shower of beautiful opalescent crystals form and snow out of solution.

|
|
|
chemplayer...
Legendary
  
Posts: 191
Registered: 25-4-2016
Location: Away from the secret island
Member Is Offline
Mood: No Mood
|
|
On sodium nitrite, we did a few experiments trying to reduce molten sodium nitrate and came up with one very unconventional method which on one
occasion seemed to work really well.
We'd got a small crucible of molten sodium nitrate, and then switched off the burner. We then kept the mixture molten via the heat of the reaction
which proceeded.
We took long strips of dried spaghetti (Barilla I think the brand was) and slowly inserted them into the molten nitrate. These immediately charred and
then burned with a hissing sound and some smoke coming off. No violent reaction, just a slow hissing and evolution of heat.
After a while we noticed that the reaction became less energetic, and so we allowed the mixture to cool and then broke up the creamy-white solid which
remained.
On addition of dilute HCl there was a vigorous reaction and a lot of NO2 gas produced from this solid!
We tried to replicate it but with mixed success - the temperature has to be exactly right! But worth playing with!
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
That is indeed an interesting procedure. Since nitrates/ites stay molten at lower temperatures when they are a mix of various alkali salts, perhaps a
mixture of sodium and potassium nitrate, heated together, would be better, if one simply desired a nitrite salt and not a particular alkali analog.
|
|
|
| Pages:
1
2 |