DrDevice
Hazard to Self
 
Posts: 74
Registered: 19-3-2012
Member Is Offline
Mood: Incompatible with carbon based lifeforms
|
|
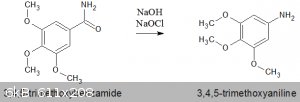
Hofmann rearrangement of 3,4,5-trimethoxybenzamide using household bleach
To support a series of experiments to obtain various phenylhydrazines, the following experiment was undertaken using a benzamide as a starting
material. This procedure is a tidied up version of the one in the Organic section of ScienceMadness.
Domestic bleach solution titred to 0.97M was used. The bleach was several months old, and from an open bottle, by the time the experiment took place.
Dissolve 6.2g (0.16 mol) NaOH in 88ml of 0.97M (0.085 mol) sodium hypochlorite solution and chill in fridge to 4C.
Add approx 40g ice.
With magnetic stirring, add 16.33g (77.3 mmol) of 3,4,5-trimethoxybenzamide all at once. Continue stirring for a further 10 minutes.
Increase the temperature to 70C over an interval of about an hour, and then add a solution of 9.2g of NaOH in 20ml water. Continue heating and
stirring at 70C for a further hour. The solution becomes a dark red-brown colour.
After the initial temperature increase, a precipitate begins to form. On removal from heat, a further mass of crystals form.
The loose fluffy product is filtered and washed with ice cold water. The product is slightly soluble in water, so some loss occurs at this time. The
filtered solid is air dried.
Measured melting point of product = 110C cf lit. 110 - 113.
Yield 3,4,5-trimethoxyaniline was 8.5g = 60%.
References
"The Hofmann Rearrangement Using Household Bleach : Synthesis of 3-Nitroaniline"(Monk, Mohan 1999),
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Thanks for the contribution. There is not much to add. I'm surprised you got such a close mp without a recrystallization. Was the product brown? Did
you synthesize the starting material yourself?
Link to discussion thread on this reaction: https://www.sciencemadness.org/whisper/viewthread.php?tid=64...
I could find some references for the Hofmann rearrangement of 3,4,5-trimethoxybenzamide:
With NaOCl:
| Quote: | | 3,4,5-Trimethoxyaniline (3) To 600 ml of 12% commercial sodium hypochlorite solution and 600 ml 2 M NaOH, compound 2 (160.0 g, 0.75 mol)was added in
portions over 30 minutes. The reactionmixture was vigorously stirred below 25 °C until TLC showed that compound 2 had disappeared; the reaction
mixture was then heated to 100 °C under stirring. After the reaction was complete, it was cooled and the solid was collected and saved. The filtrate
was extracted with CH2Cl2 (2 × 2000 ml) and the extracts were dried over anhydrous magnesium sulfate, filtered and evaporated in vacuo to give a
solid. This solid was combined the solid above and recrystallized from water to give compound 3 White crystals (109.7 g, 80% yield). mp. 111-113 C,
lit.13110-110.5 C. 1HNMR: δ5.95(s, 2H), 3.82(s, 6H), 3.77(s, 3H). MS (m/e): 183(M+). |
Cited from: An Alternate Preparation of 3,4,5-Trimethoxyphenol
Qi-Xue Qin , Jian Yang , Duo-Zhi Chen , Bo Yang , Ji Zhang
Organic Preparations and Procedures International, 2013, 45, 321-324.
DOI: 10.1080/00304948.2013.798570
From DOI: 10.3969/j.issn.0253-2417.2013.04.018 (English abstract):
| Quote: | | The optimal reaction conditions of (3,4,5-trimethoxybenzamide) was as follows: n(ammonia):n(3,4,5-trimethoxybenzoyl chloride)=4:1, reaction
temperature-5-0℃, reaction time 30 min. The optimal reaction conditions of (3,4,5-trimethoxyaniline) was as follows:
n(NaOH):n(3,4,5-trimethoxybenzamide)=9:1, n(Br2):n(3,4,5-trimethoxybenzamide)=1.15:1, rearrangement reaction temperature 40℃, rearrangement reaction
time 20 min, decarboxylation temperature 85℃, decarboxylation time 1 h, deionized water as the solvent. Yield of obtained product was 66.8%, m.p.
112.5-113.5℃, purity of obtained product was 99.0%. |
Bioorganic & Medicinal Chemistry, 2014, 22, 738-755 describes a related synthesis from 3,4,5-trimethoxybenzoic acid using the
related Curtius rearrangement. A Lossen rearrangement is described in Advanced Synthesis & Catalysis, 2013, 355, 81-86.
A very interesting strategy is used in WO2012092880. Here they nitrated 3,4,5-trimethoxybenzoic acid with HNO3 to obtain an ipso
substituted product 3,4,5-trimethoxynitrobenzene. This is then reduced to 3,4,5-trimethoxyaniline. Quite surprising that the nitration would give
nitrodecarboxylation with useful selectivity:
| Quote: | | 1,2,3-trimethoxy-5-nitrobenzene To a solution of nitric acid (20.0 mL) and acetic acid (40.0 mL) under 10 °C was added 3,4,5-trimethoxybenzoic acid
(10.0 g, 47.0 mmol) in portions. The mixture was stirred at room temperature for 30 minutes. The reaction mixture was then poured into ice-water. The
resulting precipitate was collected by filtration and washed with water, which was then recrystallized from ethanol to afford the product
1,2,3-trimethoxy-5-nitrobenzene. Yield 6.64 g, 66.1 %. 1H NMR (400 MHz, DMSO-d6) δ ppm 7.51 (s, 2H), 3.87 (s, 6H), 3.77 (s, 3H).
|
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
|