bluamine
Hazard to Others
  
Posts: 197
Registered: 17-8-2015
Member Is Offline
Mood: No Mood
|
|
Ammonia from sodium hydroxide & urea. what would be the by-product cyanide or carbonate?
Hi everyone!!
I read before some threads on the forum here claiming this reaction would give sodium carbonate, but recently i watched a Nurdrage's video about
making sodium cyanide claiming there's an alternative method (which he didn't use) to make it using both of those reactives (NaOH & CH4ON2)!!
It's only because i really respect that guy & believe he's not an amateur or beginner, that i am still comfused about this subject.
I suppose sodium cyanide is may be dangerous so i hope i will be able to avoid it.
Need your help please 
|
|
|
macckone
Dispenser of practical lab wisdom
    
Posts: 2168
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
http://www.sciencemadness.org/talk/viewthread.php?tid=19575
given sufficient hydroxide it should proceed to carbonate.
with otherwise it stops at sodium cyanide.
this is not a 100% thing as most chemical reactions there
are equilibrium involved including those with water.
small amounts of cyanide will be released in any case.
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Is cyanide a plausible by-product in aqueous solution?#
|
|
|
bluamine
Hazard to Others
  
Posts: 197
Registered: 17-8-2015
Member Is Offline
Mood: No Mood
|
|
I think the process should be done by dry distillation
|
|
|
macckone
Dispenser of practical lab wisdom
    
Posts: 2168
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
The initial step yields water, sodium cyanide and ammonia. Sodium cyanide and water will exist in equilibrium with hydrogen cyanide and sodium
hydroxide. Sodium cyanide, water, and sodium hydroxide will form sodium carbonate and ammonia but this is the slow step. A large excess of sodium
hydroxide hydrate reduces hydrogen cyanide formation.
|
|
|
Fluorite
Hazard to Others
  
Posts: 138
Registered: 26-12-2018
Location: United Arab Emirates
Member Is Offline
|
|
Yo wtf you can't make sodium cyanide by just mixing urea and NaOH! But probably you can get some cyanates and they usually decompose to ammonium
carbonate
|
|
|
Swinfi2
Hazard to Others
  
Posts: 131
Registered: 19-2-2018
Location: England
Member Is Offline
Mood: Catalytic
|
|
I'm going to say it isn't so plausible imo to get cyanide from this.
People have been making ammonia from urea and NaOH for at least a while and as far as I know none of them seem to be worried about leaving a still pot
full of sodium cyanide.
Also people have been making cyanide in different ways for at least a while and if this was a legit process it would be a super easy well known route.
(Right?)
|
|
|
ArbuzToWoda
Hazard to Self
 
Posts: 98
Registered: 15-7-2020
Member Is Offline
|
|
Obviously no cyanides are produced in this reaction. It is a very messy one, yes, leading to formation of many organic and inorganic byproducts, but
it is mostly CO2 and ammonia.
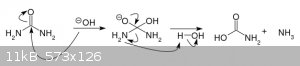
[Edited on 10-11-2020 by ArbuzToWoda]
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Biuret!
But otherwise, properly purified, the cyanate is good enough to make cyanide from.
|
|
|
macckone
Dispenser of practical lab wisdom
    
Posts: 2168
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
If the reaction is performed dry you get cyanate that rearranges to cyanide as the temperature rises.
If the reaction is performed in water you get cyanate which produces some cyanide but mostly decomposes to carbonate and ammonia.
Everything is an equilibrium, water shifts the equilibrium as does sodium hydroxide.
My initial post was incorrect, the intermediate is cyanate not cyanide.
However under dry conditions cyanide is formed.
There is another post on that specific procedure and it requires high temperature to get substantially complete conversion.
|
|
|