glassplass
Harmless

Posts: 6
Registered: 16-3-2016
Member Is Offline
Mood: No Mood
|
|
Triprolidine under birch reduction
What would happen with triprolidine molecule under "birch reduction condition"?
I need answer from experts,at least expirienced chemists...
Thanks.
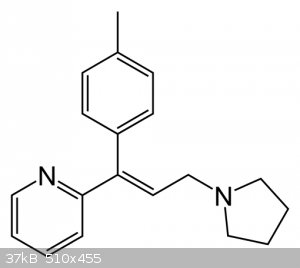
|
|
|
Nicodem
|
Thread Moved
16-3-2016 at 14:29 |
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Shooting from the hip: It would reduce the toluene group to a methyl cyclohexadiene. The pyridine group would be left as it is. I think.. depends on
the amount of Li. More base further reduction..
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
MeshPL
Hazard to Others
  
Posts: 329
Registered: 20-4-2015
Location: Universe
Member Is Offline
Mood: No Mood
|
|
That double bond conjugated with aromatic ring may also be reduced. Pyridine ring is reducable by sodium in alcohol, so it MAY be reduced in condition
of birch reduction, but I wouldn't be certain whether or not and to what extent and if it's reduction would be favoured over reduction of benzene
ring.
|
|
|