jemis
Unregistered
Posts: N/A
Registered: N/A
Member Is Offline
|
|
Fractional distillation questions
Hello,
i would like to setup a Fractional distillation appartus, but before i start i would be very grateful if you can help me on some questions
1) what would be the most appropriate ground glass joint size for distilling small quantity of organic solvents (approx ~100mL) ? 19/26, 24/29?
2) i often read that vigreux column are not efficient for Fractional distillation because of their limited theorical plates, what would you advise me
to use instead ? a humpel column with glass raschig rings ?
3) what would be the ideal length for the fractionating column knowing that i would like to distill small quantity of solvents (approx 100mL)
that's all i wanted to know
thank you alot 
[Edited on 2-3-2016 by jemis]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by jemis  | Hello,
i would like to setup a Fractional distillation appartus, but before i start i would be very grateful if you can help me on some questions
1) what would be the most appropriate ground glass joint size for distilling small quantity of organic solvents (approx ~100mL) ? 19/26, 24/29?
2) i often read that vigreux column are not efficient for Fractional distillation because of their limited theorical plates, what would you advise me
to use instead ? a liebig condenser with glass raschig rings ?
3) what would be the ideal length for the fractionating column knowing that i would like to distill small quantity of solvents (approx 100mL)
that's all i wanted to know
thank you alot 
|
These are broad questions.
1) For these small quantities I'd go 19/26. Others will undoubtedly disagree with me.
2) All other things (length and internal diameter) being equal the highest separation is obtained as follows:
packed column > Vigreux column > empty column
Packing can be Raschig rings, spheres, saddles etc.
3) There's no 'ideal' length. Separation power will not only depend on column packing and column length (for a given system to separate) but also
strongly on reflux ratio.
[Edited on 2-3-2016 by blogfast25]
|
|
|
Herr Haber
International Hazard
    
Posts: 1236
Registered: 29-1-2016
Member Is Offline
Mood: No Mood
|
|
Not necessarily 
I have a whole distillation set of Duran Rodaviss in 19/26 and as you say it's perfect for small quantities.
I use is mostly when I distill HNO3 because... well, there's teflon everywhere and being Duran I've never had a "stuck" part 
Now, if I wanted to recover solvents from my experiments or work with volumes of more than 500ml or 1L I would go for something bigger.
But then, you can always get adapters to fit a big RBF on your 19/26 setup or whatnot. I hope you are well organized and have a bottle of aspirin in
that case!
[Edited on 2-3-2016 by Herr Haber]
Damn, what am forgetting in formatting my answer?!!
[Edited on 2-3-2016 by Bert]
One stinkin' bracket added... More readable now.
[Edited on 2-3-2016 by Bert]
|
|
|
jemis
Unregistered
Posts: N/A
Registered: N/A
Member Is Offline
|
|
thanks blogfast and Herr Hebel
so a 19/26 or 24/29 is the perfect size for distilling 100ml ?
and a hempel column with raschig rings is way better than a vigreux column right ?
|
|
|
Sulaiman
International Hazard
    
Posts: 3698
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
jemis,
if RECOVERY of solvents is all you need then a simple distillation setup is sufficient.
If you want to SEPARATE solvents then it would be easier for members if you can be specific
because in my VERY limited experience,
separating solvents of different b.p. is not as easy as it sounds.
I can dis-recommend 10/19 size ... capillary tubing!
(I still like it for it's 'cuteness' though)
If I was starting from scratch then 24/29 here in UK and 24/40 in USA or whatever is cheap from China.
Buy 2x 'Distillation Kit' for a great fractionating refluxing system.
Don't forget heating, stirring, clamps, stands, cooling system,
and tubing vented to somewhere safe for vapours.
And all of the safety considerations with boiling solvents.
Turns out that the glassware is just part of the cost/consideration.
Despite the above, I hope to soon re-try stainless steel wool as packing of a fractionating column for my 10/19 kit, no reflux.
My two previous attempts resulted in a flooded column, after which, things just got worse 
for a limited number of runs, a diy setup may be sufficient ?
ethanol/moonshine distillers have some amazingly efficient diy stills,
the principles can be used at different temperature ranges.
|
|
|
Sulaiman
International Hazard
    
Posts: 3698
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
I thought that Herr Haber had gone crazy with an editing tool,
then it happened to my post.
|
|
|
jemis
Unregistered
Posts: N/A
Registered: N/A
Member Is Offline
|
|
Quote: Originally posted by Sulaiman  | jemis,
if RECOVERY of solvents is all you need then a simple distillation setup is sufficient.
If you want to SEPARATE solvents then it would be easier for members if you can be specific
because in my VERY limited experience,
separating solvents of different b.p. is not as easy as it sounds.
I can dis-recommend 10/19 size ... capillary tubing!
(I still like it for it's 'cuteness' though)
If I was starting from scratch then 24/29 here in UK and 24/40 in USA or whatever is cheap from China.
Buy 2x 'Distillation Kit' for a great fractionating refluxing system.
Don't forget heating, stirring, clamps, stands, cooling system,
and tubing vented to somewhere safe for vapours.
And all of the safety considerations with boiling solvents.
Turns out that the glassware is just part of the cost/consideration.
Despite the above, I hope to soon re-try stainless steel wool as packing of a fractionating column for my 10/19 kit, no reflux.
My two previous attempts resulted in a flooded column, after which, things just got worse 
for a limited number of runs, a diy setup may be sufficient ?
ethanol/moonshine distillers have some amazingly efficient diy stills,
the principles can be used at different temperature ranges.
|
thanks sulaiman for the response
i would to achieve a nearly complete separation
for example i would to purify small quantity of xylene with unkown impurities as much as possible, i would like also to isolate major components of
essential oils
yeah i know about the other pieces i need, i just want to know what would be the ideal setup for getting very pure compounds
as for the safetly, i'm experimenting outside and i work only with small quantity so no worries
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
24/29 is my preference for glassware joints.
A 250mm or 300mm vigreux works quite well, and is simple to use.
'Very Pure' requires more than just a distillation rig - it all depends on exactly how pure you want to go.
Edit:
Herr Haber deleted a single closing square bracket from the auto-inserted quote text. I U2U'd about it but Bert beat HH to the editing.
[Edited on 2-3-2016 by aga]
|
|
|
Herr Haber
International Hazard
    
Posts: 1236
Registered: 29-1-2016
Member Is Offline
Mood: No Mood
|
|
I have gone crazy with all the terrible gasses they made me do 1 century ago 
Thanks for Aga for his help !
I'm curious Sulaiman, why stainless steel wool? I do understand that the surface must be bigger than any other medium but is it also to distill
something in particular?
That's a very interesting idea in any case. But what about heat transfer? Steel must conduct heat quite well on top of having a bigger surface.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Without knowing the scale of your distillations, ie, the quantity to be distilled, your question is a bit like asking "what size boat should I buy for
fishing?"  And I want to only catch the most wily fish. And I want to only catch the most wily fish. 
In the US, the common sizes are: 14/20, 19/22, and 24/40. These would correspond to small, medium, and large, respectively.
Most of my distillations are in the range of 25-250 ml. For this range I prefer 19/22. But I also have a 24/40 kit for the few times I want to
distill larger amounts.
As aga says, for ultrapure separations you will need, a long or specialized column, really good packing, and a column head that will allow the tuning
of the reflux ratio. Even then you may still have 1000-10,000 ppm of impurities in the distillate.
This question is really application specific.
[Edited on 3-3-2016 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I mostly use 24/29 and buy replacement pieces that are 24/40. You can distill quantities as small as 5 mL in a 24/40 setup, but I wouldn't generally
recommend distilling anything under 25 mL in one. I have been thinking about picking up a 14/20 set for small distillations. 14/20 pieces are
generally compatible with 14/10 ones, so you can use compatible equipment for distilling quantities of less than a microliter to 500 mL.
Most professional chemists I know prefer 19/22 for home use.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
@Magpie - The OP specified the scale of distillation:
Quote: Originally posted by jemis  |
1) what would be the most appropriate ground glass joint size for distilling small quantity of organic solvents (approx ~100mL) ? 19/26, 24/29?
|
I'll agree with others here that 19/26 is probably ideal for the target quantities (100 mL in a 250 mL flask). B24 may be slightly oversized, but will
allow you to process larger quantities if that becomes a future requirement.
Quote: Originally posted by jemis  |
2) i often read that vigreux column are not efficient for Fractional distillation because of their limited theorical plates, what would you advise me
to use instead ? a humpel column with glass raschig rings ?
|
I would only use a vigreux column for rough fractionation of a larger quantity. Collecting fractions with a bp range of ca 20 *C sounds about right.
These could then be re-processed by careful fractionation in a more efficient column.
Quote: Originally posted by jemis  |
3) what would be the ideal length for the fractionating column knowing that i would like to distill small quantity of solvents (approx 100mL)
|
The type of column packing determines the height-equivalent theoretical plate (HETP). The length of the column divided by the HETP will provide the
theoretical plates of the column. To acheive separation, you'll need enough theoretcial plates and a sufficient reflux ratio. Simple packed columns
have a significantly smaller ("better") HETP than vigreux, dufton or snyder columns, and they're much cheaper too. The trade-off is that they also
tend to have a larger hold-up. I suggest looking at the section on fractional distillation in Vogel, available in the forum library.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quote: Originally posted by Magpie  | | As aga says, for ultrapure separations you will need, a long or specialized column, really good packing, and a column head that will allow the tuning
of the reflux ratio. |
!!!
I just said that it's not so simple !
The detail is all credit to you Magpie, not i.
|
|
|
jemis
Unregistered
Posts: N/A
Registered: N/A
Member Is Offline
|
|
thanks to you all!!! 
you really helped me alot unlike other places where i got either locked thread, rude responses or no responses !
time to start experimenting now 
[Edited on 2-3-2016 by jemis]
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
added to what aga said you might want to heat the column too. Bring it close to the bp of your liquid. I'd be looking at building a 14/20 system for
the small quantities you're talking about. Also you might want a non-reactive pusher liquid the boils a bit higher than your target liquid to get the
last out.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Ah, yes. Sorry.
---------------------
I edited my post to change from 14/10 to 14/20. 14/20 is probably more common and gives you another 10mm of sealing length.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
jemis
Unregistered
Posts: N/A
Registered: N/A
Member Is Offline
|
|
Quote: Originally posted by chemrox  | | added to what aga said you might want to heat the column too. Bring it close to the bp of your liquid. I'd be looking at building a 14/20 system for
the small quantities you're talking about. Also you might want a non-reactive pusher liquid the boils a bit higher than your target liquid to get the
last out. |
yes this what i want to do, but someone told it was a stupid lol
anyway thank you guys you rock!!
|
|
|
testimento
Hazard to Others
  
Posts: 351
Registered: 10-6-2013
Member Is Offline
Mood: No Mood
|
|
For efficient and high fractionation to occur one will need a column with packing and a reflux cooler with flow control before the condenser. This
allows a reflux ratio of 10:1 or more. Passive fractionation works to some extent but unless the boiling point difference is large (in scale of 30C)
and distillation speed is slow, the resulting distillate will be impure.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by jemis  |
yes this what i want to do, but someone told it was a stupid lol
anyway thank you guys you rock!! |
It would be stupid if you don't have a reflux controller on top. A column heated to near the BP, w/o a reflux controller, would act like a well
insulated steam pipe. In short, it wouldn't actually do anything!
|
|
|
jemis
Unregistered
Posts: N/A
Registered: N/A
Member Is Offline
|
|
Quote: Originally posted by blogfast25  | Quote: Originally posted by jemis  |
yes this what i want to do, but someone told it was a stupid lol
anyway thank you guys you rock!! |
It would be stupid if you don't have a reflux controller on top. A column heated to near the BP, w/o a reflux controller, would act like a well
insulated steam pipe. In short, it wouldn't actually do anything! |
i didn't find anything about reflux controller , can you explain me what it is and what it look like?
how can i heat the column near a certain bp point ?do i need a special column ?
[Edited on 4-3-2016 by jemis]
[Edited on 4-3-2016 by jemis]
[Edited on 4-3-2016 by jemis]
[Edited on 4-3-2016 by jemis]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Here's what some of these (expensive!) beauties look like:
http://www.sigmaaldrich.com/labware/glassware-catalog/distil...
http://www.sigmaaldrich.com/catalog/product/aldrich/z273120?...
http://www.sigmaaldrich.com/catalog/product/aldrich/z602876?...
It's what controls the reflux ratio R:
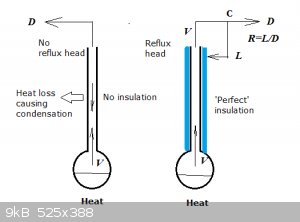
Left: 'passive' distillation. Reflux is caused by natural condensation. Typical column type is Vigreux.
Right: fractionation with reflux head allows to achieve high values of R and control them well (at point 'C', condenser flow is split into D and L).
Column is usually thermally insulated but (rarely heated, IMHO).
[Edited on 4-3-2016 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Wow ! Some of those disty heads look awesome.
Gotta get me a fraction cutter (if only i knew what it, or the 4 valves each did).
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | Wow ! Some of those disty heads look awesome.
Gotta get me a fraction cutter (if only i knew what it, or the 4 valves each did). |
You can also build one yourself: our moonshining friends have some nifty all-copper designs.
|
|
|