Dope Amine
Harmless

Posts: 40
Registered: 29-12-2011
Location: West Coast Baby
Member Is Offline
Mood: No Mood
|
|
Synthesis of Phenylacetylcarbinol by Alkyne Hydration and Subsequent Enamine formation
Input on the following is greatly appreciated... 
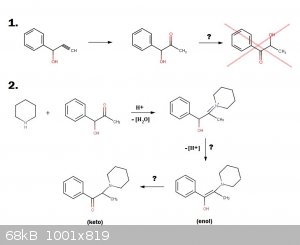
1. Firstly, in the hypothetical hydration of 1-Phenyl-2-propyn-1-ol (H2SO4, HgSO4) it is hoped that the yielded
product would be 1-hydroxy-1-phenyl-propan-2-one (phenylacetylcarbinol, PAC). BUT, the concern is that given this product is an alpha-ketol it might
possibly isomerize to 2-hydroxypropiophenone (2-HPP) in the acidic reaction solution IF 2-HPP happens to be the more thermodynamically favored
alpha-ketol arrangement. If this is the case then heating the hydration reaction would increase the isomerization to the thermodynamically favorable
product while running the reaction cold (if possible) would hopefully reduce isomerization...
Can anyone please comment on the typical reaction temperature for hydrations of alkynes (H2SO4 + HgSO4)? I
have searched high and low but have not been able to find clear details on reaction condition requirements. The hope is that the use mercury (as
opposed to just H2SO4 and heat) will lower the temperature as well as maybe the acidity required for the hydration but at the
same time not catalyze the unwanted isomerization.
Which alpha-ketol is more thermodynamically favorable, PAC or 2-HPP?
2. The next item of interest would be to react PAC with a secondary amine such as piperidine in order to form an enamine (likely with the help of
mol. sieves or azeotroping). But this product would then be an enol so it would likely have to rearrange to the keto tautomer. No, I'm not
researching cathinone analogues (especially not one that would be a pharmacological dud). Before anyone gets excited about any other N-substituted
cathinones they should realize that condensation with ammonia or a primary amine will make an imine which wouldn't set up the enol situation as
outlined here. Besides, the starting material is too expensive to be worth bothering with anyway. Assuming things went as proposed, my next step
would be to reduce the ketone to a racemic phenylpropanolamine analogue (again just a coincidence).
Do you think this enamine formation and rearrangement would go as I have outlined?
Intelligent input is greatly appreciated! THANKS!!!
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Ummm. Yesterday, I saw a mention of producing ephedrines directly, by the action of an amine upon a propenylbenzene epoxide. No reference to yield.
The epoxide is not especially difficult to produce. Alas, propenylbenzene....doesn't seem to occur in nature.
|
|
|
Skilving
Harmless

Posts: 2
Registered: 25-2-2016
Member Is Offline
Mood: No Mood
|
|
HPP will definitely be your major product pretty much no matter what you do due to resonance stabilization. But you should be able to go from HPP to
your desired product by performing an alkylation and pushing product towards water formation (acetic anhydride?).
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Dope Amine  |
BUT, the concern is that given this product is an alpha-ketol it might possibly isomerize to 2-hydroxypropiophenone (2-HPP) in the acidic reaction
solution |
actually,you should be more worried of this rearrangement,temperature control won't stop this
https://en.wikipedia.org/wiki/Meyer%E2%80%93Schuster_rearran...
the alpha ketol rearrangement is acid,base or heat induced.Because of this reason and the rearrangement mentioned above,the only way left is that you
have to protect the OH,and making the benzyl ether is the only amateur friendly way,IMHO
the protecting group can be removed later by reduction
| Quote: | | Can anyone please comment on the typical reaction temperature for hydrations of alkynes (H2SO4 + HgSO4)?
|
different books tell different tempertures.One book says 57'C while another says 60-65'C
| Quote: | Which alpha-ketol is more thermodynamically favorable, PAC or 2-HPP?
|
i would say 2-HPP
| Quote: | | The next item of interest would be to react PAC with a secondary amine such as piperidine in order to form an enamine (likely with the help of mol.
sieves or azeotroping). But this product would then be an enol so it would likely have to rearrange to the keto tautomer |
Hmmm,there is a chance that a keto-enol tautomerisation might occur,all the more reason to use a protecting group 
[Edited on 26-2-2016 by CuReUS]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2788
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
There are unconventional catalysts that will accomplish this reaction without acid:
http://pubs.rsc.org/en/content/articlelanding/2015/gc/c4gc01... (gold)
http://pubs.acs.org/doi/abs/10.1021/ja310282t (cobalt)
http://onlinelibrary.wiley.com/doi/10.1002/cctc.201400071/fu... (cobalt * salen, particularly nice)
Salen is a not-too-difficult introduction to ligands for someone who doesn't have much experience with them. Ethylenediamine is here:
http://www.sciencemadness.org/talk/viewthread.php?tid=4282
and salicylaldehyde is a classic.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
sorry to rain on your parade atara,but I don't think you read my post carefully, alpha ketol rearrangement is HEAT INDUCED
keeping that in mind , your cobalt-porphyrin and cobalt-salen will not work because both need 50-80'C for quite sometime (5-20 hours).Also the salen
method needs H2SO4 as co-catalyst and acidic conditions are required in the Co-porphyrin method in some cases
the gold reference is the only promising one out of the three,but I have a feeling that there might be a reaction between the R-AuCl and the OH group
unless the OH is protected.
while doing research on the meyer schuster rearrangement,I realised that it could be catalysed by base as well.
In that case,my previous suggestion of using benzyl ether will not work since it uses base in the protection step.Even if NaH did not cause the
rearrangement,using benzyl bromide is going to release HBr ,which would.
in fact no protection group that I know of fulfills the requirements (no acid or base in the protection or deprotection step,no acidic or basic side
products during protection) to prevent the MS rearrangement.
This is quite a difficult problem 
[Edited on 27-2-2016 by CuReUS]
|
|
|
Dope Amine
Harmless

Posts: 40
Registered: 29-12-2011
Location: West Coast Baby
Member Is Offline
Mood: No Mood
|
|
Wow! I so appreciate all of the great replies. For what it's worth, all of my subsequent reading has indicated that PAC is more thermodynamically
favored over 2-HPP. In addition to information indicating this, there's also an Alpha-ketol rearrangement wikipedia page which discusses the rules
for acyloin rearrangements and the applicable rules all point to PAC being preferred. The main concern in industry is the isomerization of L-PAC to
D-PAC, but that's got nothing to do with my goals.
CuReUS, I didn't even think about the Meyer–Schuster rearrangement being an issue. Thanks! I would love to know exactly how acidic and vigorous
the conditions are typically for that because it doesn't seem to be a clearly selective reaction. Check out this abstract from Org. Biomol. Chem.,
2009,7, 4149-4158 and I think you'll get the gist. Getting hands on the actual article would be key though:
"The Meyer–Schuster rearrangement is the conversion of propargyl alcohols into α,β-unsaturated carbonyl compounds via a formal 1,3-hydroxyl shift
and tautomerization. The major challenge associated with the Meyer–Schuster reaction is that of selectively promoting the desired rearrangement over
the myriad other reaction pathways available to propargyl alcohols. This Perspective Article features recent advances in the Meyer–Schuster
reaction, including several demonstrated techniques for improving the scope. Strengths and weaknesses of each technique are discussed, and outstanding
problems that warrant further study are highlighted. The primary motivation for research and development of the Meyer–Schuster rearrangement is as a
means of preparing α,β-unsaturated carbonyl compounds as part of a two-stage olefination strategy."
The 3 most common alkyne hydrations (non-exotic catalysts, etc.) are purely sulfuric acid which requires elevated temps, sulfuric and HgSO4
catalyst which likely requires no heat, and lastly formic acid, which may need heating or possibly not. Maybe using a weaker acid such as acetic acid
and just a drop of sulfuric acid could be helpful by creating and protonated acetic acid species which might be more selective in action. But my bet
is on using the mercury catalyst with with a minimal amount of sulfuric, hoping that the mercury will help facilitate my desired reaction over the
others.
Thanks for everybodys kind help on this mental excursion! If I could figure out some good identification spot tests then maybe I'd just run some
small test batches. The nMR testing would make things a bit pricey though...
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
that is very surprising  , could you explain how ? , could you explain how ?
| Quote: | | The 3 most common alkyne hydrations (non-exotic catalysts, etc.) are purely sulfuric acid which requires elevated temps, sulfuric and HgSO4
catalyst which likely requires no heat, and lastly formic acid, which may need heating or possibly not |
just H2SO4 ? I thought the Hg catalyst was essential in this reaction . The HgSO4 method needs 60-65'C to run
could you post the reference for using formic acid ?
|
|
|
Dope Amine
Harmless

Posts: 40
Registered: 29-12-2011
Location: West Coast Baby
Member Is Offline
Mood: No Mood
|
|
Originally alkyne hydrations were just sulfuric acid. It was later discovered that mercury could catalyze the reaction.
Alpha-ketol rearrangements: According to the "Favorskii rule," an empirical guideline with numerous exceptions, products with the carbonyl group
adjacent to a methyl group or distal to a phenyl group are favored over the corresponding isomers. PAC is the product with the carbonyl group
adjacent to a methyl AND distal to a phenyl group, and therefore favored over 2-HPP. Colard, P.; Elphimoff-Felkin, I.; Verrier, M. Bull. Soc. Chim.
Fr. 1961, 516.
With regard to formic acid:
The researchers found that dry formic acid hydrated alkynes at room temperature without any need for a transition metal catalyst. It supplies
H2O and gives off CO.
http://pubs.acs.org/doi/abs/10.1021/jo00008a058
http://pubs.acs.org/doi/abs/10.1021/jo00078a023
They say that oxygenated alkynes are resistant and do require a catalyst though. When they say "oxygenated" do they mean ketones, alcohols, ethers...
all of the above? I wish I could see the subsequent pages for examples.
|
|
|
halogen
Hazard to Others
  
Posts: 372
Registered: 18-4-2004
Member Is Offline
Mood: No Mood
|
|
Not maybe relevant, but your phrasing "It was later discovered that mercury could catalyze the reaction." made me think what else could, and lept to
mind the thought 'why can't silver?'
http://onlinelibrary.wiley.com/doi/10.1002/aoc.2918/abstract
The silver catalysis towards the hydration of terminal alkynes is explored using Silver(I) triflate (AgOTf). The reaction leads to the formation of
only Markovnikov addition product with excellent yield.
So there you go. Not everybody wants a cumulative neurotoxin.
F. de Lalande and M. Prud'homme showed that a mixture of boric oxide and sodium chloride is decomposed in a stream of dry air or oxygen at a red heat
with the evolution of chlorine.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Dope Amine  |
Alpha-ketol rearrangements: According to the "Favorskii rule," an empirical guideline with numerous exceptions, products with the carbonyl group
adjacent to a methyl group or distal to a phenyl group are favored over the corresponding isomers. PAC is the product with the carbonyl group
adjacent to a methyl AND distal to a phenyl group, and therefore favored over 2-HPP.
|
wow,then this must be the biggest trick question of the decade.I asked a lot of people which one they thought was more stable and everyone said is was
2-HPP.
good references for the formic acid hydration
Quote: Originally posted by Dope Amine  |
They say that oxygenated alkynes are resistant and do require a catalyst though. When they say "oxygenated" do they mean ketones, alcohols, ethers...
all of the above? I wish I could see the subsequent pages for examples. |
yes,and unfortunately your alcohol is similar to those examples,so you would have to use a Ru catalyst,which completely spoils the beauty of the
formic acid method 
but what about which protecting group to use ? the thought of it is giving me heartburn 
[Edited on 29-2-2016 by CuReUS]
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
the formic acid method is amazing.I wonder why no one mentioned it before
I was thinking about it and a thought entered my mind
what would happen if instead of formic acid, formamide was used along with a reducing agent like NaBH3CN or the formamide was itself used
to do a one pot reduction of the enamine formed ? (If the enamine or the imine forms at all,that is).It might not be possible to use NaBH4
as it ignites spontaneously when it comes in contact with DMF,so it might do the same with formamide.
https://en.wikipedia.org/wiki/Sodium_borohydride#Safety
(I hope others don't take this in a bad way,I just want to discuss the chemistry since its so interesting ,and drugs are not the only amines out
there.)
A method already exists to convert terminal alkynes to amines,(they use NaBH3CN to reduce the imine formed) but it needs a titanium
catalyst.If this works ,it could be an easy way to convert alkynes directly to amines.
http://pubs.acs.org/doi/abs/10.1021/ol035653%2B
[Edited on 1-3-2016 by CuReUS]
|
|
|
Polverone
Now celebrating 21 years of madness
|
Thread Split
12-3-2016 at 14:44 |
Dope Amine
Harmless

Posts: 40
Registered: 29-12-2011
Location: West Coast Baby
Member Is Offline
Mood: No Mood
|
|
halogen, I never thanked you for providing that silver triflate idea and paper. I was thrilled to try a reaction that didn't warrant such paranoia
upon work-up. Unfortunately, it doesn't work with propargyl alcohols like 1-Phenyl-2-propyn-1-ol because it generates a Meyer-Shuster rearrangement
product and other crap. For whatever reason the HgSO4/H2SO4 reaction doesn't have that problem.
I should also mention (in response to CuReUS's comment) that phenylacetylcarbinol is actually the more stable structure. 2-HPP rearranges to PAC (see
this PAPER for proof).
[Edited on 12-1-2018 by Dope Amine]
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
You might be interested in this method. Slightly different starting materials, similar products. http://www.organic-chemistry.org/abstracts/lit4/214.shtm
|
|
|
Dope Amine
Harmless

Posts: 40
Registered: 29-12-2011
Location: West Coast Baby
Member Is Offline
Mood: No Mood
|
|
Thanks. I actually just posted that paper in another thread here.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2788
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
| Quote: | | sorry to rain on your parade atara,but I don't think you read my post carefully, alpha ketol rearrangement is HEAT INDUCED |
Forgot to mention, alpha-ketol rearrangement is irrelevant on this substrate because phenylacetylcarbinol is the more stable isomer than
benzoylmethylcarbinol. See:
http://digitool.library.mcgill.ca/webclient/StreamGate?folde...
"Considerable work has been done on rearrangements of the two isomeric ketols, phenylacetyl carbinol (LXXIX) and benzoyl methyl carbinol (LXXX).
[image]
Favorskii (130) heated (LXXX) with a few drops of concentrated sulfuric acid in a sealed tube at 120-130° and obtained the isomer (LXXIX). "
The reference, 130, is Favorskii, Bull. Soc. Chim., 39, 216 (1926), if you want more details.
[Edited on 12-1-2018 by clearly_not_atara]
|
|
|