Metallus
Hazard to Others
  
Posts: 116
Registered: 16-5-2013
Member Is Offline
Mood: No Mood
|
|
Unsinkable sieve
The other day in lab I was cleaning my sieve with an ultrasound bath, when I noticed that pressing the sieve against water created an air bag
underneath which didn't allow the sieve to sink, even though air should have supposedly escaped from the several holes (large 400um) of the grit.
I painted what I did:
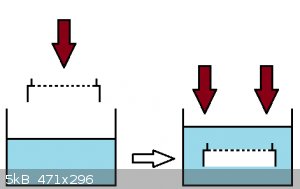
The sieve would only sink if I inclined it by a certain angle, in order to allow water to enter from the bottom sides, but if I pressed it by
maintaining the sieve parallel to the water surface, it wouldn't sink for shit.
Something tells me that this has to do with the capillar force that keeps water well "stuck" to the small holes (the sieve was wet afterall), creating
a film of water that doesn't let air pass, no matter how much I pressed.
... or am I totally off with my assumption? Why does this happen? (Just random things that occasionally happen and that are enough to amaze an
ignorant mind)
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
| Quote: | | . . . it wouldn't sink for shit. |
You could heat the water slowly and see what happens . . . ?
|
|
|
Texium
Administrator
       
Posts: 4619
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Perhaps try it again with a completely dry sieve and see if you get the same results.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Heating the water will reduce its viscosity, but now I'm unsure  if it's viscosity
trapping the air or is it surface tension or a combination of both . . . if it's viscosity
trapping the air or is it surface tension or a combination of both . . .
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
Try it with some soap in the water.
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1640
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
As others said need some surficant, bit of isopropyle, or soap.
|
|
|
Fulmen
International Hazard
    
Posts: 1725
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
I doubt viscosity is the answer as it should only decrease flow rates. Surface tension is far more likely, I assume it's the same effect that causes
wet fabrics to trap air.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
Metallus
Hazard to Others
  
Posts: 116
Registered: 16-5-2013
Member Is Offline
Mood: No Mood
|
|
Tomorrow I'll try with the dry sieve and then I'll wet it again and try adding some soap and see if this happens again.
By the way, this is not exactly a "problem", but more like a curiosity of mine. I was just surprised that no matter how much I pressed the sieve, the
air trapped inside wouldn't come out of the pores.
If I dip the sieve midway (If I don't totally drown it in water), I can see through holes and it is clearly devoid of water (Besides, I can see the
water level rise noticeably), which makes me think that there is either no water at all on the surface of the grit or that it is such a thin layer
that it's invisible to the naked eye.
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
The next step is to make a screen door for a submarine.
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Bubble Point Pressure
It is due to surface tension, and here is a page that will permit you to calculate the pressure required to force the water through the screen:
http://www.lenntech.com/library/fine/bubble/bubble-point.htm
This is used as a means of measuring screen pore sizes.
|
|
|
Metallus
Hazard to Others
  
Posts: 116
Registered: 16-5-2013
Member Is Offline
Mood: No Mood
|
|
Oh yes, it makes sense, thank you.
I didn't know this was actually used to determine pore size, interesting indeed. Thanks
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
be damned careful sonicating porous glass filters. I sonicated a fine filter in NaOH/MeOH/H2O solution and it broke up the filter. Fucking
disintegrated it. It wasn't in that long either. It only happened once but I have stopped using strong base when cleaning them in the sonic bath since
that occurred.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Metallus
Hazard to Others
  
Posts: 116
Registered: 16-5-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by chemrox  | | be damned careful sonicating porous glass filters. I sonicated a fine filter in NaOH/MeOH/H2O solution and it broke up the filter. Fucking
disintegrated it. It wasn't in that long either. It only happened once but I have stopped using strong base when cleaning them in the sonic bath since
that occurred. |
The ones I use are made of aluminium and I usually sonicate them in water, so it shouldn't be an issue.
However, I'll pay attention if I'll ever use a glass filter, thanks for the prompt.
|
|
|