enkay
Harmless

Posts: 11
Registered: 7-6-2006
Member Is Offline
Mood: No Mood
|
|
Aromatic to Cyclohexane compound
Hey friends !
Is it possible to make :
4-Aminomethyl Cyclohexane Carboxylic acid FROM 4-Aminomethyl Benzoic acid....?
No such data in beilstein available for this route. Can anyone pls help me out for this ?
[Edited on 21-8-2006 by enkay]
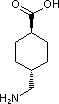
|
|
|
samdar
Harmless

Posts: 6
Registered: 11-8-2006
Member Is Offline
Mood: No Mood
|
|
no, most reagents/ reaction conditions that will reduce the benene ring(ie. raney nickel or pd carbon under pressure) will destroy your functionality.
|
|
|
ergoamide
Harmless

Posts: 33
Registered: 16-7-2006
Member Is Offline
Mood: No Mood
|
|
Hmm you could try a birch reduction maybe to get it to a diene and form there not sure how ya might gop about reducing the other double bonds, maybe
NaBH4 but i aint sure.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Well, http://www.pubmedcentral.gov/articlerender.fcgi?artid=93338 says | Quote: | | The anaerobic bacterium Syntrophus aciditrophicus metabolized benzoate in pure culture in the absence of hydrogen-utilizing partners or terminal
electron acceptors. The pure culture of S. aciditrophicus produced approximately 0.5 mol of cyclohexane carboxylate and 1.5 mol of acetate per mol of
benzoate |
The start of an article using platinum and haloacid, you'll need to get the full article to see if it works for you
http://pubs.acs.org/cgi-bin/abstract.cgi/jacsat/1936/58/i09/...
|
|
|
ziqquratu
Hazard to Others
  
Posts: 385
Registered: 15-11-2002
Member Is Offline
Mood: No Mood
|
|
Article attached:
J. H. Brown, H. W. Durand, and C. S. Marvel (1936) "The Reduction of Aromatic Compounds with Hydrogen and a Platinum Oxide- Platinum Black Catalyst in
the Presence of Halogen Acid", J. Am. Chem. Soc, 58(9), 1594 - 1596
Attachment: Aromatic Reduction with Pd+HX.pdf (316kB)
This file has been downloaded 1199 times
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Ah, thank you ziqquratu! Not only does that look as if would be useful to enkay, it might do the trick when one needs a small amount of some
cycloalkanes. It also appears that short reaction times could be used to remove a halogen from an aromatic, although you'd probably want to recycle
unreacted material.
|
|
|