DrDevice
Hazard to Self
 
Posts: 75
Registered: 19-3-2012
Member Is Offline
Mood: Incompatible with carbon based lifeforms
|
|
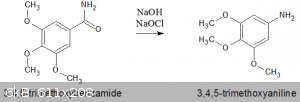
Hofmann Rearrangement of 3,4,5-trimethoxybenzamide
Another experiment in a series to obtain various phenylhydrazines, this time with a benzamide as a starting material.
Drawing heavily on the procedure outlined in "The Hofmann Rearrangement Using Household Bleach : Synthesis of 3-Nitroaniline"(Monk, Mohan 1999), I
attempted the reaction using 3,4,5-trimethoxybenzamide. There are other procedures in the literature for this compound (see note below), but the ease
of using household bleach was a preferred approach.
Dissolve 3.2g (0.08mol) NaOH in 24ml of 12.5% w/v sodium hypochlorite solution. I calculated this to be 1.7M, but made no attempt to titre this more
accurately. 24ml = 0.04mol NaOCl.
This solution was placed in an ice bath, 40g ice added & 40ml cold water. To this was added 8.0 g (0.038 mol) of 3,4,5-trimethoxybenzamide, with
stirring.
(I initially tried this step without the ice cooling, but the reaction was quite exothermic and a black gunk resulted. Referring to the other papers,
ice was used and then heating afterwards, and this approach seemed successful.)
Temperature was then slowly increased to 60C over 1 hour. After 1 hour, a solution of 5g NaOH in 5ml water was added, and the temperature raised to
70C with stirring continuing for another hour.
During this time, precipitate begins to form. On removal from heat, a mass of crystals form. This was filtered and washed with ice cold water. I
believe some product did still dissolve during this washing, but no attempt was made to recover any dissolved product.
After drying 4.16g material was obtained. MW of 3,4,5-trimethoxyaniline is 183.2, so yield of 22.7mmol ie 60%, compared to the 80% yield in the
referenced paper.
I didn't add the NaHSO3 that was in the paper, as it seemed there was copious yield. I will attempt the experiment again with this step at
some point to see what difference it makes ie to improve yield. I will also attempt recrystallization from ethanol.
I used this material in a later diazo experiment and it reacted in the typical way with sodium nitrite and sodium sulfite, so I am reasonably
confident I obtained the desired result.
I have just gone looking for the exact reference to other papers - I thought was in OrgSyn, but buggered if I can find it now. I'll keep looking &
will post it if I find it.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
http://www.prepchem.com/synthesis-of-3-nitroaniline/ ?
|
|
|
SunriseSunset
Hazard to Self
 
Posts: 82
Registered: 9-6-2015
Location: USA
Member Is Offline
Mood: No Mood
|
|
Nice workup! You said it yourself, you probably lost a little bit of yield through the filter wash. Why not just extract into a solvent then dry and
salt out or evaporate to residue?
[Edited on 25-1-2016 by SunriseSunset]
Why do chemists call helium, curium and barium the medical elements?
because if you cant helium or curium, you barium! - Heimerdinger
|
|
|
DrDevice
Hazard to Self
 
Posts: 75
Registered: 19-3-2012
Member Is Offline
Mood: Incompatible with carbon based lifeforms
|
|
Thanks for the comment. Yes, next time I will try a solvent approach. Ethanol has worked well for me in the past in other hydroxy and methoxy
substituted anilines.
It's just been too damn hot to get back into the workshop...
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
In nature there cannot exist 12,5% NaOCl solution. There can be "approx 10% solution", "I've just measured it to be a 7.3% solution", or "week ago it
was a 5.7% solution".
You provided neither melting point measurement, nor crystalls, nor any other proof that you've obtained the correct compound. I believe you've got a
mixture of carbamate and benzoate. I have no idea what's the purpose of the second portion of NaOH you've used, but I can definitely say the purpose
of NaHSO3 - to hydrolyze the carbamate and destroy any chlorine left in the solution. You know, you can't just a add table salt instead of sodium
nitrite into the reaction and hope the result will be the same.
|
|
|
DrMethyl
Harmless

Posts: 34
Registered: 23-11-2015
Member Is Offline
Mood: No Mood
|
|
Hello, nice work indeed !
Dont worry, you often have less yield than in the paper.. byko3y is right take care about the conc. of NaOCl. Because Im lazy about bleach titration I
always use calcium hypochlorite to be sure.
Did you check your final compound ? Did you try to dissolve into dil HCl to see wheter it dissolves ?
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
@byko3y: what is the "bleaching powder" of commerce?
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
SunriseSunset
Hazard to Self
 
Posts: 82
Registered: 9-6-2015
Location: USA
Member Is Offline
Mood: No Mood
|
|
Would using calcium hypochlorite actually work?! I wondered about that but assumed it wont. Haven't got a chance to try it.
Why do chemists call helium, curium and barium the medical elements?
because if you cant helium or curium, you barium! - Heimerdinger
|
|
|
SunriseSunset
Hazard to Self
 
Posts: 82
Registered: 9-6-2015
Location: USA
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by byko3y  | | I believe you've got a mixture of carbamate and benzoate. I have no idea what's the purpose of the second portion of NaOH you've used, but I can
definitely say the purpose of NaHSO3 - to hydrolyze the carbamate and destroy any chlorine left in the solution. You know, you can't just a add table
salt instead of sodium nitrite into the reaction and hope the result will be the same. |
I'm curious why you'd say this when there's a decent number of references i could cite for you using simply a 2nd portion of NaOH or KOH.
They all pretty much start off with 2 part NaOH and 1 part NaOCl 1 part amide, then the writeups either include NaOH, KOH or 10% aq NaHCO3.
So unless I'm mistaken, he used the 2nd portion of NaOH to compensate the role of the NaHCO3. Which should of worked fine.
Why do chemists call helium, curium and barium the medical elements?
because if you cant helium or curium, you barium! - Heimerdinger
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
chemrox, I don't understand your question, did you want to ask something like "what is in fact sold as bleaching powder?"? Sodium and
calcium hypochlorites degrade over time, so when it says on the label "12,5% sodium hypochlorite" - it means "not higher than 12,5% hypochlorite". In
fact in our shops the labels say "higher than 15% sodium hypochlorite", that means "it was higher than 15% sodium hypochlorite few month ago". AFAIK,
the only non-gaseous product of decomposition of sodium hypochlorite is NaCl.
SunriseSunset, you are right, heating the carbamate with alkali hydroxide will hydrolyze the carbamate, but there was no
rationalization in the OP's message.
Both calcium hypochlorite and sodium hypochlorite work the same, but they both decompose over time.
|
|
|
DrDevice
Hazard to Self
 
Posts: 75
Registered: 19-3-2012
Member Is Offline
Mood: Incompatible with carbon based lifeforms
|
|
An influence on my procedure was the OrgSyn article on the preparation of 4-aminoveratrole, which in the second step also involves a Hofmann
rearrangement, where a second amount of NaOH is added.
That procedure generates NaOCl directly with chlorine passed into NaOH solution - however, it was easier to do my home experiment with a bleach
solution, and hence my use of the first referenced paper. No, I don't know what the purpose of the second NaOH addition in the 4-aminoveratrole paper
is for.
byko3y, your point regarding bleach molarity is taken, and I'll be titrating next time. And adding NaHSO3.
Regarding the verification of the product, no I don't have a melting point etc. My evidence is indirect - a diazotization with NaNO2 was performed,
with strong colours developing on addition of the nitrite. A near-exact amount of nitrite was required for the amount of aniline (as tested by
starch/iodide), as is typical for a diazotization.
Subsequent addition of sodium sulfite resulted in a strongly yellow coloured precipitate typical of a diazosulphonate.
I'm just learning here, guys. My professional/educational background is electrical engineering and I'm only just getting back into a chemistry hobby I
abandoned decades ago. Mostly developing lab technique right now and only gradually picking up the deeper theory...
|
|
|
DrDevice
Hazard to Self
 
Posts: 75
Registered: 19-3-2012
Member Is Offline
Mood: Incompatible with carbon based lifeforms
|
|
I followed up on a couple of comments made in an earlier part of the thread, and repeated the experiment.
I titrated the bleach & determined it was 0.97M, and used an amount accordingly.
I built a melting point apparatus (a block of aluminium with a hole to place a thermometer into, heated with my heater/stirrer) and measured a quite
sharp melting point of 110C (lit 110 - 114).
Subsequent diazotization & addition of sulfite to form a diazosulfonate worked smoothly, as before, as did a following reduction to the sulfonic
acid using sodium dithionite. I'll post a procedure sometime.
I'm pretty sure I ended up with what I intended
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Thanks for sharing. I would very much like to see it posted in the Prepublication forum section, once you get all the data and write a report.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Quote: Originally posted by byko3y  | | chemrox, I don't understand your question, did you want to ask something like "what is in fact sold as bleaching powder?"?
|
I didn't want to digress so much. This is an actual organic chemistry thread and a good one. I would buy
3,4,5-trimethoxy benzaldehyde because I can and have. But this is about a rearrangement and I'd like to see it proceed and get optimized. But your
question was what was I asking so here: I don't know bleaching powder. What is it? (I just learned from you it is Ca-hypochorite) why can't it be a
solid, i.e. why is it so unstable? How much Ca-hypochorite is in "bleaching powder? So what I'm asking here is why is Ca-hypochorite unstable? But
this is off-topic so if you want to say it could be in general chemistry or u2u. Thanks.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
Calcium hypochloride is as unstable as any hypochlorite is. It is less unstable when mixed (dilluted) with CaCl2 and kept at low temperature, also
some compounds are more stable than others, still they degrade over time if stored at r.t./water vapors/light.
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Reference Information
The Hofmann Rearrangement Using Household Bleach: Synthesis of 3-Nitroaniline
Keith A. Monk and Ram S. Mohan
J. Chem. Educ.
1999, 76 (12), p 1717
DOI: 10.1021/ed076p1717
Abstract
The Hofmann rearrangement is an important example of a rearrangement reaction that is discussed in most sophomore organic chemistry texts. Yet very
few examples of this reaction can be found in lab texts. We have developed a simple experiment that involves the Hofmann rearrangement of
3-nitrobenzamide to give 3-nitroaniline using household bleach. The synthesis of 3-nitrobenzamide by nitration of benzamide and the subsequent Hofmann
rearrangement can be carried out in two-and-a-half hours, making this a new and simple two-step reaction sequence for the organic laboratory.
Attachment: The Hofmann Rearrangement Using Household Bleach- Synthesis of 3-Nitroaniline.pdf (16kB)
This file has been downloaded 1091 times
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
Boffis
International Hazard
    
Posts: 1900
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Sorry I'm a bit late to the party but while researching something completely different (purpurogallin) I came across a detailed synthetic account of
exactly the OP's requirement. If anyone is still interested check out:
J. Chem. Soc, [1951] p1318-1325; Critchlow et al; Purpurpogallin VI; The Mechanism of oxidation of pyrogallol
In the experimental section they describe the preparation of various starting materials in detail including exactly the OP's requirement and its
conversion to 1,2,3-trimethoxy-5-iodobenzene via diazotization. So all the details you need; even the preparation of sodium hypochlorite from KMnO4
and HCl!!! What more could you desire?
|
|
|
DrDevice
Hazard to Self
 
Posts: 75
Registered: 19-3-2012
Member Is Offline
Mood: Incompatible with carbon based lifeforms
|
|
Thanks for the further information, Boffis. However, I am actually pursuing hydrazines, rather than (eg) 3,4,5-trimethoxybenzaldehyde, so the amine
was what I was after to form a diazo then reduce to a phenylhydrazines. And the use of liquid bleach is easier than faffing around with gaseous
chlorine.
I'll put up my subsequent phenylhydrazine sulfonic acid prep some time Real Soon now...
The comments about the stability of calcium hypochlorite are interesting. Is the stability concern for solutions or the solid state? Calcium
hypochlorite solid I thought was stable for years, and thus (as per DrMethyl's comment) safer to rely on for OCl- content. All the doomsday preppers
out there swear by the long-term stability of solid calcium hypochlorite for water purification, and who are we to argue with them 
BTW, I've put a tidied up version of the procedure in Prepublications.
|
|
|
Boffis
International Hazard
    
Posts: 1900
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@DrDevice, you need to read the experimental section as it describes specifically the hofmann degradation you are trying to carry out ie the
preparation of the trimethoxyaniline never mind the title subject. If you don't read you won't learn much.
|
|
|