CitricAcid
Harmless

Posts: 28
Registered: 13-12-2015
Member Is Offline
Mood: No Mood
|
|
Acid strength test?
According to a video by NurdRage on making chloroform, chloroform is decomposed by sodium hydroxide to give formic acid. I want to try and produce
some formic acid that way,but is there any way to test the concentration of the acid? I know that a good way to test to see whether or not sulfuric
acid is concentrated to 98% is to add it to sugar and see whether or not it turns to carbon,but is there any way to test to see whether or not formic
acid is concentrated to 85%?
|
|
|
Detonationology
Hazard to Others
  
Posts: 362
Registered: 5-5-2015
Location: Deep South
Member Is Offline
Mood: Electrophillic
|
|
| Quote: |
...the chloroform produced decomposes under strongly alkaline solutions... chloroform reacts with sodium hydroxide to produce formic acid and sodium
chloride. |
I haven't quite found any data on this quite yet, but I can definitely recommend a method of producing formic acid from anhydrous glycerin and oxalic
acid. Here is a link,and TheChemiKid has made a video of this process. Here is an extremely simplified version of the scheme. In step 1, oxalic acid
and glycerin react at elevated temperatures to yield glyceryl monoxalate and water. In step 2, the oxalate part of the compound is decarboxylated to
yield glyceryl monaldehyde and CO2. The final step is the hydrolysis of the aldehyde to yield formic acid and glycerin, which is regenerated from
this process.
[Edited on 12-16-2015 by Detonationology]
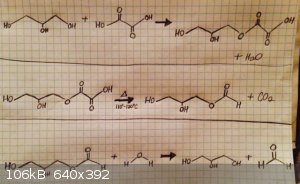
“There are no differences but differences of degree between different degrees of difference and no difference.” ― William James
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
I imagine this would proceed via dichlorocarbene (look up hazards of this). Look out for carbon monoxide and the possibility of phosgene.
I'd be very careful when trying this out. Carbenes are frisky.
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
CitricAcid
Harmless

Posts: 28
Registered: 13-12-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Detonationology  | | Quote: |
...the chloroform produced decomposes under strongly alkaline solutions... chloroform reacts with sodium hydroxide to produce formic acid and sodium
chloride. |
I haven't quite found any data on this quite yet, but I can definitely recommend a method of producing formic acid from anhydrous glycerin and oxalic
acid. Here is a link,and TheChemiKid has made a video of this process. Here is an extremely simplified version of the scheme. In step 1, oxalic acid
and glycerin react at elevated temperatures to yield glyceryl monoxalate and water. In step 2, the oxalate part of the compound is decarboxylated to
yield glyceryl monaldehyde and CO2. The final step is the hydrolysis of the aldehyde to yield formic acid and glycerin, which is regenerated from
this process.
[Edited on 12-16-2015 by Detonationology] |
What is that at the end of the reaction scheme? The formula for formic acid is CH2O2.
Nevermind,I looked it up,the results came back with formaldehyde,I think you made a mistake there  . Thanks for drawing the sequence out though. . Thanks for drawing the sequence out though.
[Edited on 16-12-2015 by CitricAcid]
|
|
|
CitricAcid
Harmless

Posts: 28
Registered: 13-12-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Ozone  | I imagine this would proceed via dichlorocarbene (look up hazards of this). Look out for carbon monoxide and the possibility of phosgene.
I'd be very careful when trying this out. Carbenes are frisky.
O3 |
How would I get adequate amounts of dichlorocarbene?
"Look our for carbon monoxide and the possibility of phosgene"
I'm assuming it reacts with oxygen in the air to produce phosgene,is there any other alternative?
[Edited on 16-12-2015 by CitricAcid]
|
|
|
UC235
National Hazard
   
Posts: 565
Registered: 28-12-2014
Member Is Offline
Mood: No Mood
|
|
Chloroform does indeed react with aqueous sodium hydroxide. In the presence of a secondary solvent like methanol so that the reaction mixture is
homogenous, this reaction can get rather agressive as it is very exothermic.
The reaction proceeds by intermediacy of dichlorocarbene. This is not isolatable. The hydroxide deprotonates chloroform to trichloromethyl anion,
which eliminates Cl- to charge-neutral but very reactive dichlorocarbene. This inserts itself into a water O-H bond forming "dichloromethanol" which
eliminates HCl to formyl chloride and is hydrolyzed and deprotonated to formate.
As a source of formate, the whole process is massively inefficient. 79ml (119g) of chloroform requires 160g of sodium hydroxide for complete reaction.
After boiling down the solution and discarding 174g of NaCl, you would get 68g of sodium formate (in a perfect world, which this is not).
As for turning it back into formic acid, you can't use sulfuric acid unless you want to die of CO inhalation. I'm not even sure what the best approach
would be. Phosphoric acid, maybe. The acid forms a high boiling 77% azeotrope at 107C which will be functionally impossible to sepatate from water
using distillation on a lab scale. Not counting water, a perfect yield would be 46g. I would be stunned to get half that.
The process using glycerol and oxalic acid posted above will be far simpler and higher yielding.
If you want to know the concentration of formic acid, you could try to find a density table and take very accurate density measurements at the
reference temperature. But really, you should learn how to titrate for a definitive answer.
[Edited on 16-12-2015 by UC235]
|
|
|
Detonationology
Hazard to Others
  
Posts: 362
Registered: 5-5-2015
Location: Deep South
Member Is Offline
Mood: Electrophillic
|
|
Quote: Originally posted by CitricAcid  | What is that at the end of the reaction scheme? The formula for formic acid is CH2O2.
Nevermind,I looked it up,the results came back with formaldehyde,I think you made a mistake there  . Thanks for drawing the sequence out though. . Thanks for drawing the sequence out though.
[Edited on 16-12-2015 by CitricAcid] |
Thank you for pointing that out. We can all get ahead of ourselves sometimes. The product of this reaction is indeed formic acid, I just forgot one
measly O at the end. That's all it takes to screw you in chemistry, I guess.
“There are no differences but differences of degree between different degrees of difference and no difference.” ― William James
|
|
|
ave369
Eastern European Lady of Mad Science
   
Posts: 596
Registered: 8-7-2015
Location: No Location
Member Is Offline
Mood: No Mood
|
|
For sulfuric acid the test is,whether it carbonizes sugar. For nitric acid - whether it sets a narrow strip of rubber on fire. For the other acids, no
strength tests exist.
Smells like ammonia....
|
|
|
Boffis
International Hazard
    
Posts: 1867
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Somewhere on the SM Forum I have already posted a patent (try US 3718545) about the azeotropic concentration of formic acid to >98% using heptane
or petroleum ether of the correct boiling range. I didn't try it because I found an OTC supplier of 98% formic acid and its cheap.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Is there no Titration possible, seeing as formc acid is, erm, an acid ?
|
|
|
Detonationology
Hazard to Others
  
Posts: 362
Registered: 5-5-2015
Location: Deep South
Member Is Offline
Mood: Electrophillic
|
|
I think this on the right track...
“There are no differences but differences of degree between different degrees of difference and no difference.” ― William James
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
For those that don't know, Titration is really useful and lets you find out how 'strong' an acid or base is by using an indicator, like
phenolpthalein.
In Summary :-
you stick a measured quantity of your formic acid in a beaker, make an exact concentration of say NaOH solution (or some base that reacts in a known
ratio with your acid), add a coloured acid/base indicator to the acid in the beaker, then drip the basic solution into the beaker, drop by drop, until
the indicator changes colour.
That is what a Burette is for.
From the volume of the base added you can calculate how many moles of acid there were.
From that and the volume of acid you started with, you can calculate the original concentration (viz Strength) of your acid.
It's actually a lot easier to do than describe.
http://chemwiki.ucdavis.edu/Analytical_Chemistry/Quantitativ...
|
|
|
CitricAcid
Harmless

Posts: 28
Registered: 13-12-2015
Member Is Offline
Mood: No Mood
|
|
Idea
Seeing as sulfuric acid reacts with formic acid to produce carbon monoxide,a gas,couldn't an experiment be done that determines the amount of gas
produced where sulfuric acid of ,say,98% concentration is added to an equal volume of 98% formic acid? Then,the data from the "control" experiment can
be compared to data from experiments where an unknown concentration of formic acid is used with 98% sulfuric acid?
Edit:I forgot, but for the discussion,let's say that 10Ml of 98% formic and sulfuric acid is used,both for the control experiment and for the formic
acid with an unknown concentration.
Let's say that I find the amount of gas produced with 98% formic and sulfuric acid,which is represented as X,then I test a sample of formic acid with
unknown concentration using 98% sulfuric acid,and the amount of gas produced is Y.
Y/X= Concentration of unknown acid sample (Maybe?).
Of course, the measurement will be off by a few %s since 98% acid was used in the control experiment rather than 100%,but I think the plan is pretty
sound.
I got the idea after doing some reading about a setup where hydrogen peroxide and household bleach are used to determine the concentration of the
NaClO in the bleach,let me know what you think.
[Edited on 16-12-2015 by CitricAcid]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
CO is not nice, and gasses are harder to measure than dissolved chemical species in a solution.
When in solution, they don't escape so easily.
|
|
|
CitricAcid
Harmless

Posts: 28
Registered: 13-12-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | CO is not nice, and gasses are harder to measure than dissolved chemical species in a solution.
When in solution, they don't escape so easily. |
Let's say that the control experiment is done in a round bottom flask with vinyl tubing leading into an upside-down graduated cylinder filled with
water. By "Amount" I meant the volume of carbon monoxide gas that is made.
Edit: I'm talking about a setup similar to what the maker of the linked video does.
https://www.youtube.com/watch?v=j-PrAczOGb0
[Edited on 16-12-2015 by CitricAcid]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Does Titration sound too difficult ?
CHRIS25 used syringes 'cos he had no burette.
Got pretty close to 0.1 accuracy if i recall correctly ...
|
|
|
DraconicAcid
International Hazard
    
Posts: 4334
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
And if you can't get phenolphthalein, you can use cabbage juice as an indicator.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
RED cabbage juice.
Tut Tut Draconic.
Not all Cabbages are created alike.
Edit:
The juice from a red cabbage is a remarkably good pH indicator though.
[Edited on 16-12-2015 by aga]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4334
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Good point, but I'm not an expert in cabbages. I mean, ew.......they're cabbages.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Red cabbages should always be on hand for eating, chemistry, dye, offensive flatulence and even as a weapon if you run out of cannon balls.
Cannot ever have enough red cabbages IMHO.
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
The best thing would be HPLC. A BioRad Aminex HPX-87H (300 mm) with a RID, eluted with 0.008N H2SO4 at about 0.6 mL/min will give you formic at about
14 minutes (linear up to about 2000 ppmw, LOQ ~40 ppmw, on-column). Oxalic elutes much earlier.
Barring that, the pKa is 3.75, so the end point would probably be somewhere around 6. So, either methyl red or bromothymol blue should give end points
that are pretty close.
With red cabbage juice, you could shoot for purple (lavender, actually, which is the problem), but I've found end points to be hard to really nail
with this indicator (there are just too many of them). Also, you have to use it fresh. It tends to turn into brown muck.
If you have a pH meter, though, you're good to go. Just put the probe in there and titrate by volume (say 0.1 N NaOH) and plot the pH vs titrant as
you go. The center of the plataeu should be very close to 3.75. The endpoint should also be obvious.
If your resolution is fine enough (as your patience allows), you may also see additional features that will indicate other acidic protons (mixed
acids).
If time permits, I'll titrate some tomorrow, for reference.
O3
[Edited on 17-12-2015 by Ozone]
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
j_sum1
Administrator
       
Posts: 6326
Registered: 4-10-2014
Location: At home
Member Is Online
Mood: Most of the ducks are in a row
|
|
A seasonal alternative to red cabbage juice:
http://www.compoundchem.com/2015advent/2015advent17/
I had no idea.
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Stillage from Elderberry wine, adjusted to various pH, for your enjoyment.

-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
ave369
Eastern European Lady of Mad Science
   
Posts: 596
Registered: 8-7-2015
Location: No Location
Member Is Offline
Mood: No Mood
|
|
Guys, we have a whole wiki article about DIY pH indicators!
http://www.sciencemadness.org/smwiki/index.php/Anthocyanin
More to the topic, I have access to heptane and no use for it except stove lighting aid. I mean, of course, distilling Et2O out of Quick-Start car
sprays. I may try the experiment with heptane and formic acid.
Concerning titrations, a Janet syringe (a giant syringe which is easy to find in drugstores and medical supplies shops) makes a fine burette if you
don't have a real one. Of course, this is a better option, but not the only one: I titrated HCl with normal syringes, filling them with the titrant
and emptying them several times.
[Edited on 17-12-2015 by ave369]
Smells like ammonia....
|
|
|