CitricAcid
Harmless

Posts: 28
Registered: 13-12-2015
Member Is Offline
Mood: No Mood
|
|
Pen Ink Separation?
I am a newb to this forum,so hopefully I am not off to a bad start. I would like to know how I can separate pen ink into its components. I am not
talking about the experiment where a piece of filter paper is used to separate ink into the colors that make it up,I am talking about separating out
solvents,dye compounds,etc. For instance,supposed that pen ink contains (insert dye chemical here) dissolved in something like ether. Let's suppose
that a certain brand of pen ink contains 3 dyes and 4 different solvents(just as an example),I would like to know a way to separate out all the
solvents and dyes,preferably with as little contamination as possible.
[Edited on 14-12-2015 by CitricAcid]
|
|
|
Dark Alchemist
Harmless

Posts: 38
Registered: 14-11-2015
Location: Behind you. No really look!!!
Member Is Offline
Mood: No Mood
|
|
suspicion. just a hunch that you are after the more chemically destructive
Stuff contained in pen ink eg nerve agents or toxic compounds? Other wise why would you be bothering hmmmm....... This may be over suspicion just a
strange request is it not easier to obtain these compounds a different way?
[Edited on 14-12-2015 by Dark Alchemist]
[Edited on 14-12-2015 by Dark Alchemist]
If your not on a government watch list by now you should be ashamed of yourself.
|
|
|
Volanschemia
Hazard to Others
  
Posts: 340
Registered: 16-1-2015
Location: Victoria, Australia
Member Is Offline
Mood: Pretty much all of them!
|
|
He can't possibly just be interested in the process and science of the extraction?
I'm not saying there's no possibility of nefarious intent, but it seems early to jump to conclusions.
"The chemists are a strange class of mortals, impelled by an almost insane impulse to seek their pleasures amid smoke and
vapor, soot and flame, poisons and poverty; yet among all these evils I seem to live so sweetly that may I die if I were to change places with the
Persian king" - Johann Joachim Becher, 1635 to 1682.
|
|
|
Agari
Banned
Posts: 160
Registered: 8-10-2015
Location: The Amine Group
Member Is Offline
Mood: Lowest Oxidation State
|
|
Quote: Originally posted by Dark Alchemist  | suspicion. just a hunch that you are after the more chemically destructive
Stuff contained in pen ink eg nerve agents or toxic compounds? |
If pen ink actually contained nerve agent precursors,there would have been an attack similar to the Tokyo Subway Sarin Release of 1995 in the
UK,U.S.,or Canada by now. That being said,I think there is more to the story. A few searches turned up that a solvent that is sometimes used in the
dye industry is also one step away from mustard gas:
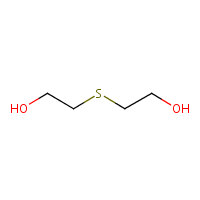
Thiodiglycol.
And the structure of mustard gas is:
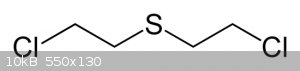
I sincerely doubt that you can actually obtain enough sulfur mustard from thiodiglycol from ink to kill any large number of people, sulfur mustard is
also hydrolyzed back to thiodiglycol,so I think we can rule out chemical weapons.
I believe that the hydrolysis/conversion equilibrium is C4H10O2S + 2 HCl <--> C4H8Cl2S + H2O
Probably a bad idea to post a virtual how-to on making sulfur mustard,but I have actually seen a few nerve agent threads from 2004 on the forum.
[Edited on 14-12-2015 by Agari]
Element Collection Status:
Elements Acquired: 21/91
Latest: Lead (Pb)
Quantity: 12g
-----------------------------------------------------
|
|
|
Volanschemia
Hazard to Others
  
Posts: 340
Registered: 16-1-2015
Location: Victoria, Australia
Member Is Offline
Mood: Pretty much all of them!
|
|
You can also generate small amounts of carbonyl chloride from decomposing trichloromethane made from bleach and acetone.
The truth is, you can make many harmful compounds from many many OTC products.
Also Agari, could you please scale down those pictures? When they are over a certain size it makes the webpage grow out of proportion.
"The chemists are a strange class of mortals, impelled by an almost insane impulse to seek their pleasures amid smoke and
vapor, soot and flame, poisons and poverty; yet among all these evils I seem to live so sweetly that may I die if I were to change places with the
Persian king" - Johann Joachim Becher, 1635 to 1682.
|
|
|
Dark Alchemist
Harmless

Posts: 38
Registered: 14-11-2015
Location: Behind you. No really look!!!
Member Is Offline
Mood: No Mood
|
|
Sorry I apologise for jumping to conclusions right of the bat.
If people jumped to conclusions every time I asked a question that could be used maliciously I would have a very big head ache.....yo what dis nigga
want conc HNO3 for? Hey wat dis terrorist want PETN for?
I just saw this and immediately thought why on earth would you want to do that!
Then again there is as he explained the love of science so again citric acid I apologise that was off of me and a poor way to welcome you.
Just out of curiosity why do you want to do that is it out of a need for a specific chemical or just to see if you can because you can?
And holy crap that's a big diagram.... I get the emphasis but I am not blind....yet
[Edited on 14-12-2015 by Dark Alchemist]
If your not on a government watch list by now you should be ashamed of yourself.
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Chromatography is still the best bet. Here, though, you'll want to use column chromatography: https://en.wikipedia.org/wiki/Column_chromatography
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
Assuming you have a pretty sizable quantity of ink, you could gently boil off all of the solvents(I don't know that you'd find more than one or two)
and collect the liquid portion using a distillation apparatus, to be further purified by fractional distillation or chromatography. Then it's just a
matter of separating the pigments/dyes by their selective solubility in various solvents, or as stated above, by column chromatography.
|
|
|
Agari
Banned
Posts: 160
Registered: 8-10-2015
Location: The Amine Group
Member Is Offline
Mood: Lowest Oxidation State
|
|
The pictures should be fine now. I found a better diagram for thiodiglycol online,so I uploaded that instead of the old one.
[Edited on 14-12-2015 by Agari]
Element Collection Status:
Elements Acquired: 21/91
Latest: Lead (Pb)
Quantity: 12g
-----------------------------------------------------
|
|
|
CitricAcid
Harmless

Posts: 28
Registered: 13-12-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Amos  | | Assuming you have a pretty sizable quantity of ink, you could gently boil off all of the solvents(I don't know that you'd find more than one or two)
and collect the liquid portion using a distillation apparatus, to be further purified by fractional distillation or chromatography. Then it's just a
matter of separating the pigments/dyes by their selective solubility in various solvents, or as stated above, by column chromatography.
|
I suspect that some of the components can form azeotropes,I will read more into column chromatography though.
|
|
|
CitricAcid
Harmless

Posts: 28
Registered: 13-12-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Dark Alchemist  |
Just out of curiosity why do you want to do that is it out of a need for a specific chemical or just to see if you can because you can?
|
I assume that the solvents in pen ink also form azeotropes,so I figured I might try to separate them,I don't have a fractional distillation
column..yet. While at it,I might as well see what exactly is in the ink.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
For really important scientific analyses like this, i always start off by adding a lot of Ethanol to see what happens.
|
|
|
Agari
Banned
Posts: 160
Registered: 8-10-2015
Location: The Amine Group
Member Is Offline
Mood: Lowest Oxidation State
|
|
Quote: Originally posted by CitricAcid  |
I assume that the solvents in pen ink also form azeotropes,so I figured I might try to separate them,I don't have a fractional distillation
column..yet. |
Then column chromatography is your best bet,like said in a previous post.
Element Collection Status:
Elements Acquired: 21/91
Latest: Lead (Pb)
Quantity: 12g
-----------------------------------------------------
|
|
|
CitricAcid
Harmless

Posts: 28
Registered: 13-12-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | | For really important scientific analyses like this, i always start off by adding a lot of Ethanol to see what happens. |
And do you often trash your glassware? 
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Of course not !
No need for a Glass when the EtOH arrives in a Tin.
|
|
|
Mabus
Wiki Master
  
Posts: 238
Registered: 3-11-2013
Member Is Offline
Mood: Energetic
|
|
Quote: Originally posted by aga  | | For really important scientific analyses like this, i always start off by adding a lot of Ethanol to see what happens. |
I do the same thing at the local pub 
|
|
|
Agari
Banned
Posts: 160
Registered: 8-10-2015
Location: The Amine Group
Member Is Offline
Mood: Lowest Oxidation State
|
|
Pffft,drunken ethanol peasants! Everyone knows that aqueous sodium hypochlorite mixed with sodium hydroxide is the true beverage for formal occasions
such as relaxation at pubs!
[Edited on 15-12-2015 by Agari]
Element Collection Status:
Elements Acquired: 21/91
Latest: Lead (Pb)
Quantity: 12g
-----------------------------------------------------
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Lizard Alien beverages are incompatible with human physiology.
Do not encourage it amongst them : your Nest Status will reduce.
[Edited on 15-12-2015 by aga]
|
|
|
Fulmen
International Hazard
    
Posts: 1718
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
I find the question meaningless. And it all boils down to the number of constituents and their widely different properties. You can have anything from
volatile solvents to non-volatile compounds with quite similar properties. And unless you know exactly what you're looking for, how will you know how
to separate them? And then there's the identification of whatever you find, how do you determine what they really are? So unless you're looking for a
specific compound or know the composition you're left with some wild stabs in the dark. Without knowing more about your goal it's hard to give you
better advice.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Reformatted for readability :-
Quote: Originally posted by Fulmen  | I find the question meaningless.
It all boils down to the number of constituents and their widely different properties.
You can have anything from volatile solvents to non-volatile compounds with quite similar properties.
Unless you know exactly what you're looking for, how will you know how to separate them ?
Then there's the identification of whatever you find, how do you determine what they really are ?
So unless you're looking for a specific compound or know the composition you're left with some wild stabs in the dark.
Without knowing more about your goal it's hard to give you better advice. |
Reads easier, better, nicer no ?
The power of a blank line every now and again ...
|
|
|
Texium
Administrator
       
Posts: 4586
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Column chromatography is probably the only feasible way of separating the dye components, but it's a rather difficult technique and requires expensive
equipment (the column and the silica, which must be a fine enough grade to be used for chromatography). I have not done column chromatography before
as I do not have the equipment for it, but I have heard that it can be a tedious and rather frustrating process. CitricAcid, if you are new to home
chemistry you might want to try some other simpler stuff before jumping into column chromatography.
Another possible option if you still want to work on the ink project would be to try solvent extraction. Perhaps try adding an immiscible solvent to
the ink and see what it will dissolve, and then separate the phases.
|
|
|
CitricAcid
Harmless

Posts: 28
Registered: 13-12-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by zts16  | CitricAcid, if you are new to home chemistry you might want to try some other simpler stuff before jumping into column chromatography.
|
I haven't tried it yet,but let's say that I decide to postpone the chromatography experiment. Preferably,I would like to be able to look at this
thread when I do decide to do column chromatography.
I'll try chloroform in the meantime.
[Edited on 16-12-2015 by CitricAcid]
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
You might try paper chromatography... also, chloroform is pretty easy to make as long as you measure right and make sure everything is cold enough.
I can't imagine that column chromatography is all that hard really - you first do TLC to find the best solvent mixture, and then separate in a column,
taking a bunch of samples, which you run through TLC to verify purity... it looks like it would be pretty straightforward and easy as long as you
don't take any shortcuts, but then again, I have never actually performed column chromatography, so perhaps it is harder than it looks.
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
With colored compounds, it's really simple to tell which fractions to collect. This would make column chromatography quite easy with pen ink.
|
|
|