KristofferVN
Harmless

Posts: 1
Registered: 22-11-2015
Member Is Offline
Mood: No Mood
|
|
Acquiring Uranium/Uranium compounds
Hi!
I am looking to acquire smaller amounts of some uranium compounds, preferably uranylnitrate. Uranium metal could also be a viable option.
I know that a good way to acquite such, would be through United Nuclear, Ebay or chemical suppliers for that matter.
Problem is that as a European, I can't seem to find anyone who are willing to ship such compounds within Europe, and especially not to individuals.
Does anyone happen to have a useable source for Uranium or its compounds?
Websites would be especially useful, but if any individuals would be willing to sell me some from their own collection, that would be helpful as well.
Thanks.
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Quote: Originally posted by KristofferVN  | Hi!
I am looking to acquire smaller amounts of some uranium compounds, preferably uranylnitrate. Uranium metal could also be a viable option.
I know that a good way to acquite such, would be through United Nuclear, Ebay or chemical suppliers for that matter.
Problem is that as a European, I can't seem to find anyone who are willing to ship such compounds within Europe, and especially not to individuals.
Does anyone happen to have a useable source for Uranium or its compounds?
Websites would be especially useful, but if any individuals would be willing to sell me some from their own collection, that would be helpful as well.
Thanks. |
Are you willing to undertake extracting them from uranium ore? Shannon & Sons is a German based outfit offering high grade Hartenstein pitchblende
for about $140 a kilo. I looked into acquiring some, but having it shipped to the U.S. from Germany was a problem.
This website discusses how to uranium ore processing:
https://carlwillis.wordpress.com/2008/02/20/uranium-chemistr...
Alternatively you could arrange with a US member here to do the purchase and forward it to you - say, the UN uranium metal, from which many compounds
could be prepared.
If you wish to discuss this U2U me.
|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
https://carlwillis.wordpress.com/2008/02/20/uranium-chemistr...
"The connection to the server was reset while the page was loading"
I have tried that link for weeks always getting the same message. In fact lately I get it on an overwhelming number of links searching Google for
various unrelated technical topics. Far more often than what I would call normal, so much so I have to wonder if some kind of massive blocking is
being caused by I have no idea who. Even get it searching for such mundane topics as Pic DDS projects. Can you if you have no problem going there copy
this info and post it here. Waited for years for United Nuclear to ever finish their pages on the subject, where they promised to do pages on solvent
extraction yet never did.
"Science is the belief in the ignorance of the experts" Richard Feynman
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
No problems accessing the link from here. Attached is a pdf copy of the website for those who can't access it directly. The pictures refused to copy
over nicely with the text, so they are in the accompanying zipped folder.
Attachment: Uranium Chemistry.pdf (297kB)
This file has been downloaded 1155 times
Attachment: Uranium Compounds.zip (5.2MB)
This file has been downloaded 782 times
|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
Thanks. Just tried it again same as always. I do not know if it is my ISP blocking or what but I get this at many locations. Far too often to be
coincidence.
"Science is the belief in the ignorance of the experts" Richard Feynman
|
|
|
urenthesage
Hazard to Self
 
Posts: 77
Registered: 21-2-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by KristofferVN  | Hi!
I am looking to acquire smaller amounts of some uranium compounds, preferably uranylnitrate. Uranium metal could also be a viable option.
I know that a good way to acquite such, would be through United Nuclear, Ebay or chemical suppliers for that matter.
Problem is that as a European, I can't seem to find anyone who are willing to ship such compounds within Europe, and especially not to individuals.
Does anyone happen to have a useable source for Uranium or its compounds?
Websites would be especially useful, but if any individuals would be willing to sell me some from their own collection, that would be helpful as well.
Thanks. |
http://www.ebay.ca/itm/331772776256?_trksid=p2055119.m1438.l...
This guy is selling Uranyl acetate at 33 euros/20 grams. This might be what you need as the acetate can easily be converted to the nitrate with a
little acid.
|
|
|
Great
Harmless

Posts: 34
Registered: 8-2-2016
Member Is Offline
Mood: No Mood
|
|
Theres a member here, Mario, that sells Uranyl compounds (among many other things) from Europe. If you cant find his thread, Ill U2U you his email.
|
|
|
Joe Skulan
Harmless

Posts: 21
Registered: 16-3-2016
Member Is Offline
Mood: No Mood
|
|
It may not be the most efficient starting material, but I've isolated uranium from dinosaur bone, as a byproduct of my ongoing attempt to get the
radium. The bone I'm using is 0.1-0.3% U, but I have a lot of it, and it's exempt from most regulations.
|
|
|
phlogiston
International Hazard
    
Posts: 1379
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
Thats a pretty nice accomplishment, Joe Skulan. I would not mind hearing more on how you did it and what the actual yield was.
Are dinosaur bones specifically different in this respect than other bones? And if so, am I correct to assume that this is related to the fossilation
process rather than due to a high level of incorporation of uranium in the bone during life of the animal?
Another source may be bentonite kitty litter. It supposedly contains enough uranium to set off radiation alarms when transported in large quantities
and it is cheap. But good luck extracting and concentrating it, especially since the bentonite itself probably also has a non-negligable affinity for
it.
[Edited on 17-3-2016 by phlogiston]
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
Joe Skulan
Harmless

Posts: 21
Registered: 16-3-2016
Member Is Offline
Mood: No Mood
|
|
There's nothing special about dinosaur bone, except that the bones are large and spongy and that helps them trap U (and Th). Apart from that it
depends on the composition of the groundwater around the bones, not the particular kind of bone.
It all happens post-depositionally (I don't like the term "fossilize" because it implies that there is some process necessary for a thing to become a
fossil, beyond just getting old. It gets causation backward: a thing becomes a fossil if it is not destroyed, and no particular "process" is required
for something not to be destroyed. Most fossil bone (and wood) is just the original material impregnated with minerals). There seem to be two main
mechanisms: 1. Direct precipitation of U minerals from groundwater in bone cavities, and 2. Sorption onto surface of hydroxyapatite nanocrystals. Both
processes may be helped by the reducing environment created around a slowly decomposing carcass. Sorption probably accounts for most of the U in bone.
Underground dams of ground fish bone are used to trap actinides in groundwater in this way. The bone I'm using is from the ilium of a Triceratops from
the Hell Creek Fm. Radioactive bone is fairly rare in that formation, but this appears to have been deposited near a major river draining the
proto-Rockes to the west, which would have supplied U and Th from weathered granite.
Starting with 1000 grams of bone, I got about 2.1 grams of U, in a mix of transition metal oxides.
As I said, my goal is to isolate the radium (based on a strong gamma signal from 214Bi, which is near the end of the decay chain, the 238U in the bone
seems to be at least close to equilibrium with its daughter nuclides). I probably will post something on that, as I need some help with it.
The pic is of coffinite (a U silicate- light gray) with embedded pyrite crystals (dark gray) in a Haversian canal in a dinosaur bone.
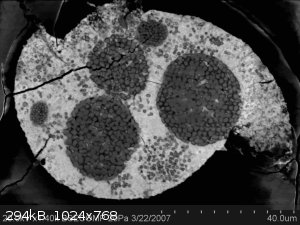
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by phlogiston  |
Another source may be bentonite kitty litter. It supposedly contains enough uranium to set off radiation alarms when transported in large quantities
and it is cheap. But good luck extracting and concentrating it, especially since the bentonite itself probably also has a non-negligable affinity for
it.
|
Yes. Also kaolin, acc. this source:
http://health.phys.iit.edu/extended_archive/9511/msg00461.ht...
Years ago I had a chance of buying 25 g of Uraninite concentrate from eBay but not being a radiochemist I was afraid to radio contaminate my lab in
the process of extracting a pure uranium compound from it. So I didn't buy it. Still have some lingering regrets about that...
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Joe Skulan  |
The pic is of coffinite (a U silicate- light gray) with embedded pyrite crystals (dark gray) in a Haversian canal in a dinosaur bone.
|
Impressive. How do you know which is which?
|
|
|
Joe Skulan
Harmless

Posts: 21
Registered: 16-3-2016
Member Is Offline
Mood: No Mood
|
|
By XRD. But in that image the lighter the color the greater the density, and that alone tells you that whatever is surrounding the pyrite has a much
higher density than iron, which usually is the densest thing you encounter.
|
|
|