noitanima
Harmless

Posts: 6
Registered: 23-10-2015
Location: Japan
Member Is Offline
Mood: No Mood
|
|
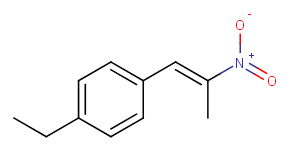
Is 4-ethylphenyl-2-nitropropene liquid in room temperature?
Excuse me.
I use machine translation because English isn't understood.
4-ethylbenzaldehyde 5.4mL, nitroethane 3.6mL and n-butylamine 0.4mL were mixed.
It was heated by 100 ℃ for several hours.
Acetic acid 1mL and n-butylamine 0.2mL have been added.
It was heated for several hours.
Reaction liquid became blackish brown.
IPA in several mL has been added.
It was cooled in about minus 20 ℃.
(A bottle was soaked in the liquid HFC-152a taken out of gas duster.)
A crystal separated out.
While being still cold, liquid was thrown away.
The same thing was performed twice.
But when it'll be room temperature, a crystal in IPA has disappeared.
Is 4-ethylphenyl-2-nitropropene liquid in room temperature?
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2788
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
The more likely answer is that your nitropropene is more soluble in warm iPrOH than cold iPrOH. Many things have solubility that changes with
temperature.
If the crystal had melted and not dissolved, you'd see it as a layer at the bottom.
[Edited on 24-10-2015 by clearly_not_atara]
|
|
|
noitanima
Harmless

Posts: 6
Registered: 23-10-2015
Location: Japan
Member Is Offline
Mood: No Mood
|
|
Thank you very much.
Several tests were done.
When I skimmed a crystal in cold IPA and loaded a paper towel down, it melted immediately.
Next I heated liquid by 100 ℃ and made IPA evaporate.
When remaining liquid was cooled to about minus 20 degrees, it froze.
When its solid was put on a paper towel again, it melted immediately.
I think so it's unrelated to the solubility to IPA.
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
A crystal? Try an alternate synthesis. There are many. For purity, bigger crystals are better. Less total surface area.
This reaction usually produces a solid block of bright yellow, orange, or red crystals.
Omit the acetic acid. Use a minimal amount of alcohol (ethanol might be better), and conduct the reaction by leaving the reactants at 120F, for a 7
to 10 days. While protecting from air and light. Allow to cool naturally, and let the product sit for a few days, at room temperature. This
sequence usually produces large crystals, in high yield. Expect a solid block, or a semi-solid block of crystals.
This approach has worked well for me.
If you have to have your product sooner, you could try Heinzelmann's technique. He uses Butylamine as a catalyst, runs the reaction in refluxing
toluene, and drives the reaction by separating out, water as it is formed. A Dean-Stark trap is used. http://www.google.com/patents/US2557051
It is also possible to run this reaction neat, or without solvents. Seems like you can just take your major reactants and microwave them, to produce
a good yield almost instantly. Keeping in mind, that you are working with a nitro-alkane, and it does have some explosive potential.
Use the search function. There will be many examples.
[Edited on 24-10-2015 by zed]
[Edited on 24-10-2015 by zed]
|
|
|
noitanima
Harmless

Posts: 6
Registered: 23-10-2015
Location: Japan
Member Is Offline
Mood: No Mood
|
|
I receive advice and thank you very much.
I'm trying a reaction of various benzaldehyde analog and nitroethane.
10 kinds were tried, but the one from which solid nitroalkene was obtained in room temperature was 3 kinds.
The one from which a solid crystal was obtained in room temperature
* 2,4-difluorobenzaldehyde + nitroethane
* 2,5-difluorobenzaldehyde + nitroethane
* 2,6-difluorobenzaldehyde + nitroethane
The one from which a solid crystal was obtained when it was cooled in the minus 20 ℃
* 4-ethylbenzaldehyde + nitroethane
The one which failed to get a crystal
* 2,3-difluorobenzaldehyde + nitroethane
* 3,5-difluorobenzaldehyde + nitroethane
* 3-chlorobenzaldehyde + nitroethane
* 3-methylbenzaldehyde + nitroethane
The one which failed to get a crystal, (When changing the way, there is a possibility which succeeds.)
* 3,4-difluorobenzaldehyde + nitroethane
* 2,4-dimethylbenzaldehyde + nitroethane
When halogen, alkyl and trifluoromethyl group stuck to meta (3) position of a benzene ring, phenyl nitropropene seems to be often liquid in room
temperature.
When the following thesis is seen, this hypothesis seems right.
https://www.mdpi.com/2076-3417/2/1/114/pdf
If there is something which becomes crystalline in room temperature after 4th in the upper list, please tell me.
Postscript
I found that 3-Amino-1-propanol is a good catalyst compared with n-butylamine recently.
3-Amino-1-propanol is more than 4 times as quick at a change with the color of the reaction liquid as n-butylamine.
It's more difficult for 3-Amino-1-propanol(bp 188 ℃) to evaporate than n-butylamine(bp 77 ℃).
When reaction solution is orange if n-butylamine is used, the drop of water in the reaction solution is clear.
When reaction solution is orange if 3-Amino-1-propanol is used, the drop of water in the reaction solution is orange.
There is a possibility that 2-Aminoethanol is more strong.
But 2-Aminoethanol is designated as "Deleterious Substances" by Japanese law.
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Back when my friends and I, were working with nitropropenyl benzenes..... One of my very experienced colleagues, told me... that ideal conditions for
producing them, varied from precursor to precursor. No single method of synthesis, seemed ideal for all cases.
As such, the method of Heinzelmann, wherein the reaction is driven, by physically removing water as it is formed, might be a useful means to employ.
The collected water proves the reaction, has...or hasn't taken place. At any rate, this is usually a high yield reaction. There should be, a lot of
product.
As for, the final products being liquids, rather than solids.......maybe.
Keep in mind, that like any crystallization, this crystallization may not be immediate.
I have had such products, stubbornly refuse to crystallize. Even though they were reasonably pure, and were known to be solids at room temperature.
Without a seed crystal to kick things off, it is hard to know, whether you are truly dealing with a liquid, or just a solid...that is merely refusing
to crystallize.
Heinzelmann, also utilizes vacuum distillation of as a means of purification of his product. The idea scares me a little. But how will you achieve a
pure product, if your oil refuses to crystalize?
Your link......doesn't seem to work. https://www.mdpi.com/2076-3417/2/1/114/pdf
[Edited on 28-10-2015 by zed]
[Edited on 29-10-2015 by zed]
|
|
|
MeshPL
Hazard to Others
  
Posts: 329
Registered: 20-4-2015
Location: Universe
Member Is Offline
Mood: No Mood
|
|
Do you plan to make drugs? 4-ethylphenyl-2-nitropropene is one reduction step from substituted amphetamine with ethyl attached to aromatic ring. Not
sure if it would have any psychoactive proprieties.
All other nitropropenyl aryls are also suspicious.
Out of curiosity, did you manage to determine yields of different reactions? Those might be pretty interesting...
|
|
|
noitanima
Harmless

Posts: 6
Registered: 23-10-2015
Location: Japan
Member Is Offline
Mood: No Mood
|
|
| Quote: |
Your link......doesn't seem to work. |
The PDF file can be read by my PC.
The title of the thesis is "A Study of Fluorinated β-Nitrostyrenes as Antimicrobial Agents".
| Quote: |
As such, the method of Heinzelmann, wherein the reaction is driven, by physically removing water as it is formed, might be a useful means to employ.
|
Thank you very much.
Unfortunately, I don't possess Dean-Stark apparatus.
I succeeded in crystallization of 3,4-difluorophenyl-2-nitropropene recently.
The following chemical was mixed and it was heated for more than 6 hours by about 100 ℃.
* 3,4-difluorobenzaldehyde (Tokyo Chemical Industry D2340) 2.2mL
* Nitroethane (Tokyo Chemical Industry N0198) 1.8mL
* 3-Amino-1-propanol (Tokyo Chemical Industry A0438) 0.4mL
This crystal was a solid matter in room temperature.
But the yield is quite low.
A catalyst will be changed and reexecuted from now on.
|
|
|
noitanima
Harmless

Posts: 6
Registered: 23-10-2015
Location: Japan
Member Is Offline
Mood: No Mood
|
|
| Quote: |
Do you plan to make drugs? |
Yes.
I'll make a legal stimulant in Japan.
Regulation of a drug became severe from July, 2014 in Japan.
Once a month, controlled substance is added.
The following substance is regulated.
* 2-fluoroamphetamine
* 3-fluoroamphetamine
* 4-fluoroamphetamine
* 2-fluoromethamphetamine
* 3-fluoromethamphetamine
* 4-fluoromethamphetamine
* 4-chloroamphetamine
* 4-methylamphetamine
* Etilamfetamine.
But the following substance isn't regulated yet.
* Difluoroamphetamines.
* Difluoromethamphetamines.
* 3-chloroamphetamine
* 3-methylamphetamine
* 4-ethylamphetamine
* Fluoroetilamfetamines.
The raw material of a compound of non-regulation is expensive.
[Edited on 12-11-2015 by noitanima]
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Some sort of Dean Stark setup isn't hard to construct, and in a pinch, simple distillation might do. Just distill off solvent, with a regular
distillation set up, and return it to your reaction flask, minus the accumulated water at the bottom.
I have always had the expectation of high yields for these reactions. In fact if the yield isn't high, it becomes much more difficult to crystallize
or otherwise isolate your products. High yields, saturate your mother liquor with product, and force crystallization. Otherwise those
Beta-nitropropenyl benzenes, are quite soluble in things like aliphatic-alcohols and acids, and probably Nitroethane itself.
I would search diligently for an approach that produces a higher yield. What you are doing, isn't working very well.
Many variants exist, for reaction conditions. Though the papers might be in English, and it may be irksome to translate them. In the past, I have
sometimes had the problem, of needing to access papers in Russian, or Japanese. Not easy to translate.
Failing that, there are specific pathways that have been successful routes to some of your desired products, that do not involve Nitropropenes.
Notably, Fluoro-substituted phenyl-2-propanones may be produced by the reaction of Fluoro-substituted diazonium salts, with iso-propenyl-acetate.
Some explosion hazards may exist.
Also of note. Some of these 2-propanones might be constructed from benzaldehydes, via the Darzens condensation.
https://en.wikipedia.org/wiki/Darzens_reaction
I assume you have legal authorization for this work.
Here in the U.S., such work is difficult for many of us to do.
Nitroethane isn't easily acquired here anymore.
[Edited on 12-11-2015 by zed]
[Edited on 12-11-2015 by zed]
[Edited on 13-11-2015 by zed]
|
|
|
noitanima
Harmless

Posts: 6
Registered: 23-10-2015
Location: Japan
Member Is Offline
Mood: No Mood
|
|
The price of Dean-Stark apparatus was 700 dollars, so I gave up buying it.
I'll try dehydrating one instead of it by a molecular sieve.
Quote: Originally posted by zed  | Failing that, there are specific pathways that have been successful routes to some of your desired products, that do not involve Nitropropenes.
Notably, Fluoro-substituted phenyl-2-propanones may be produced by the reaction of Fluoro-substituted diazonium salts, with iso-propenyl-acetate.
Also of note. Some of these 2-propanones might be constructed from benzaldehydes, via the Darzens condensation.
|
Thank you very much for your useful information.
But unfortunately it's difficult for me.
I'll learn more chemistry from now on.
| Quote: | | I assume you have legal authorization for this work. |
My work doesn't need legal permission.
| Quote: | Here in the U.S., such work is difficult for many of us to do.
Nitroethane isn't easily acquired here anymore. |
It is interesting.
American regulations, it is tough.
Acquisition of piperonal and acetic anhydride is restricted in Japan.
Possession is prohibited by P2P and ephedrine (more than 10 % of density).
But the purchase of nitromethane, nitroethane, benzaldehyde and fluoroP2Ps is easy.
I got knowledge about 2,6-difluoroamphetamine.
I reducing 2,6-difluorophenyl-2-nitropropene of 10 mmol (2 grams) using Red-Al.
A free base of 2,6-difluoroamphetamine was a solid matter.
I poured hexane 30mL, but hardly dissolved.
But when a hexane was heated until boiling point, much dissolved.
HCl solution in diethyl ether was poured into a free base, but the crystallization is impossible.
This material hydrochloride is hygroscopic like amphetamine hydrochloride.
Wet hydrochloride, when smoking, there was no psychoactivity.
Also, a free base of 2,4-difluoroamphetamine was liquid.
There is probably psychoactivity.
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Well, this ain't exactly a healthy pursuit.
That being said, the standard crystallization method, involves bubbling anhydrous HCl gas, through an anhydrous solution, containing the free amine.
|
|
|
DrMethyl
Harmless

Posts: 34
Registered: 23-11-2015
Member Is Offline
Mood: No Mood
|
|
Hello,
when you said some substrate did not work like the trifluoromethylated one, I think some substrate are deactivated by the electronegativity of the
fluorine and if your compound melt as soon as it touches the towel/filter I think its because the reaction is not finished because some target
substrates require more time to complete especially if you did it without heating. Heat will greatly reduce reaction time.
For the perfect reaction IMO :
1 eq aldehyde + 1.2 (or more)eq nitroalkane + 0.1 eq primary amine catalyst (methylamine is the best, high yield on near every substrate) + 0.1-0.2
eq. acetic acid to quench the amine + solvent (methanol is the best, i'll explain why)
Now the mixture is refluxed overnight, reaction can be checked with TLC. When the reaction is over, the flask is put in the freezer. Now if you
choosed methanol you have some benefit : the product is less soluble in MeOH it will crystallise easier, you can use a little more solvent to be
comfortable. Sometimes if small amount of MeOH is used you can see the nitropropene starting to crystallise when the solution is still refluxing. MeOH
will also dissolve the formed water unlike toluene for example.
Now if you tried all odd tricks to get crystals such as scratching with a spatula, you can simply pour water in it and after freezing, the cake is
washed with plenty water to remove R-NH2*AcOH and unreacted R-NO2 then with cold MeOH to get rid of the impurities. Finally the crystals can be
air-dried.
Could you post the method you used with all yield for each substrate please ? I am curious to see the impact of the substituent on the overall yield.
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Once again, this is not a very healthy pursuit, especially for a chemist. Having an unlimited supply of amphetamines, usually leads to disaster.
Unless of course, you are immune to temptation.
Better to get high, via the practice of yoga. Cheap! Efficient! And, without the truly terrible, and painful, amphetamine "comedown".
Motoyama, wrote an interesting book on the subject, called "Theory of the Chakras". Might be available in Japanese.
http://www.abebooks.com/servlet/BookDetailsPL?bi=17024284615...
[Edited on 16-12-2015 by zed]
[Edited on 16-12-2015 by zed]
|
|
|
|