deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
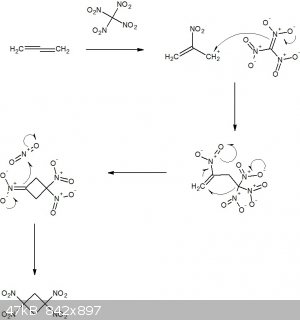
Hypothetical reaction between propadiene and tetranitromethane
I've drawn up the following hypothetical mechanism for a reaction between propadiene and tetranitromethane and would appreciate some constructive
criticism, please.
Apologies for the 'paper chemistry', I was just having a bit of fun on the breakfast table this morning.
|
|
|
kecskesajt
Hazard to Others
  
Posts: 299
Registered: 7-12-2014
Location: Hungary
Member Is Offline
Mood: No Mood
|
|
!!!FREEMASON COMPOUND!!!
Seriusly, might work but combining tetranitromethane with reducing agent is not a great idea.
Isn't breaking C=N bond difficult?
|
|
|
j_sum1
Administrator
       
Posts: 6326
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Life must be interesting inside your brain deltaH. I don't know where you get these ideas.
That sure is a weird-looking molecule. I wonder what its properties are. I am going to guess reasonably energetic.
http://www.chemspider.com/Chemical-Structure.9854991.html
There is a paper authored by someone named "Flippen-Anderson", which sounds like an attempt to swear politely.
http://scripts.iucr.org/cgi-bin/paper?hh0587
I would be really intrigued if you got this to work.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
p70 of Organic Chemistry of Explosives by Jai Prakash Agrawal, Robert Hodgson has this to say about 1,1,3,3-tetranitrocyclobutane:

********************************************************
Tetranitromethane is extremely toxic, not something I'd fiddle with, but it can be made from the exhaustive nitration of acetylene in the presence of
a little mercury as catalyst. Propadiene is readily available in the form of MAPP gas along with its isomer methyl acetylene and propane, the latter
being inert for wet chemical reactions, so it got me thinking...
I wonder if TNCB could be made by simply bubbling MAPP gas through 100% HNO3 containing mercury nitrate with a good scrubber to capture all the NOx
that would be evolved?
Perhaps just like the acetylene process for making tetranitromethane, this would also require a subsequent treatment with conc. sulfuric acid to drive
the reaction to completion and yield the product.
Probably dangerous wishful thinking, though the if it could be made to work, could be a very low-cost route to an attractive energetic.
[Edited on 18-10-2015 by deltaH]
|
|
|