Sniffity
Hazard to Self
 
Posts: 70
Registered: 27-12-2014
Member Is Offline
Mood: No Mood
|
|
Reaction mechanism of TCCA with HCl
Hey,
So I'm thinking about carrying out this simple chlorine production experiment; in which TCCA is reacted with hydrochloric acid to produce chlorine
gas.
I understand how to carry out the reaction in practice, but I'd like to get a grip on the theory behind it.
I've been trying to look for the reaction mechanism between TCCA and HCl and have so far failed to find one. My theory is that the N-chloroamines will
work as follows:
1.- The nitrogen will be protonated by the HCl, thus gaining a slightly positive charge.
2.- The chlorine will donate some of it's positive charge to the nitrogen to stabilize it.
3.- The chloride ion liberated from the HCl in step 1 will now perform a nucleophilic attack on the chlorine.
4.- Cl2 leaves, and the N-chloroamine has turned into a simple amine.
I was able to find this paper, which suggests the role of N-chloroamines as oxidizers, but does not confirm my reaction mechanism.
http://pubs.acs.org/doi/pdf/10.1021/op010103h
So: Is my proposed reaction mechanism correct? If not: Where can I find the proper reaction mechanism for this?
Also: What's the correct structure for an isocyanurate ion? Can't seem to find it anywhere.
Other things I've read:
Isocyanurate ions are actually hydrolized to form HOCl, is this true? Can't seem to find any basis for this?
-Perhaps the oxygen is protonated, and then the positive charge is passed over to the nitrogen via resonance? This just seems a little odd: Shouldn't
the product after the reaction with HCl be basically trichloroisocyanuric acid with all the chlorines replaced with Hs?
Also: Anyone got a proper scientific paper, which I can reference, in regards to the synthesis of Cl2 using TCCA and HCl? Or explaining what is
actually an isocyanurate ion?
This thing's driving me mad D: Any help will be really apprecaited
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
N-Cl bond is always shifted to nitrogen side. pKa of HCl is -7, which means that it is completely dissociated. For chloramine pka=14 and pkb=15, which
is close to that of water. I'm sure the chloramide has similar dissociation constants. Thus, the chloroamine is mostly protonated by the strong acid.
Attack of chlorammonium by nucleophile leads either to displacement of one chlorine atom with another (products and reactants are the same, reaction
is unfavorable because the nitrogen is shielded with H and Cl atoms from 4 sides) or to displacement of ammonium with chlorine by attack on
chlorammonium's chlorine
[Cl]- -> [Cl-NH3]+
Cl-Cl NH3
Chlorocyanuric acids are slowly hydrolyzed to HOCl by water.
Sodium dichloroisocyanurate
For cyanuric acid the dissociation constants are pKa1 = 6.88; pKa2 = 11.40; pKa3 = 13.5 (for comparision pKa of water is 15.7; pka of ammonia is
9.25).
[Edited on 15-10-2015 by byko3y]
|
|
|
Sniffity
Hazard to Self
 
Posts: 70
Registered: 27-12-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by byko3y  | N-Cl bond is always shifted to nitrogen side. pKa of HCl is -7, which means that it is completely dissociated. For chloramine pka=14 and pkb=15, which
is close to that of water. I'm sure the chloramide has similar dissociation constants. Thus, the chloroamine is mostly protonated by the strong acid.
Attack of chlorammonium by nucleophile leads either to displacement of one chlorine atom with another (products and reactants are the same, reaction
is unfavorable because the nitrogen is shielded with H and Cl atoms from 4 sides) or to displacement of ammonium with chlorine by attack on
chlorammonium's chlorine
[Cl]- -> [Cl-NH3]+
Cl-Cl NH3
Chlorocyanuric acids are slowly hydrolyzed to HOCl by water.
Sodium dichloroisocyanurate
For cyanuric acid the dissociation constants are pKa1 = 6.88; pKa2 = 11.40; pKa3 = 13.5 (for comparision pKa of water is 15.7; pka of ammonia is
9.25).
[Edited on 15-10-2015 by byko3y] |
First of all, thanks for your reply 
Two parts:
First in regards to the reaction mechanism:
I was thinking of the N-Cl bond: Is it actually shifted to the N side? I mean: Shouldn't the chlorine pull the electrons stronger than the nitrogen,
thus shifting the bond to the chlorine? (Then again: Nitrogen does have a lone pair, which could be vulnerable to protonotion).
I can see the chloroamine's nitrogen being protonated. But what follows? I mean:
Option 1: Something clearly has to leave that nitrogen, and it could either be Cl+ or H+. My way of seeing it is the H+ will leave, given that Cl+ is
not a good leaving group.
Option 2: If we look at the protonated chloramine as a chlorammonium: Cl- has a full electron shell, as does the chlorine in the chloramine. How would
it be able to attack it? Even if the chloramine chlorine donates electron density to the nitrogen: It would have to actually donate an electron pair
to be vulnerable to attack by the Cl-, wouldn't it?
Second: In regards to the sodium dichloroisocyanurate:
I've read references to a sodium TRIchloroisocyanurate, but can't seem to find it. Any ideas on this?
How are the ions of TCCA formed anyhow? What has to happen for an ion to be formed?
Third: Would you happen to have a source for the hydrolysis of TCCA to HOCl in the presence of water? It'd really help! D:
[Edited on 15-10-2015 by Sniffity]
[Edited on 15-10-2015 by Sniffity]
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
Ammonia is a leaving group here.
|
|
|
Sniffity
Hazard to Self
 
Posts: 70
Registered: 27-12-2014
Member Is Offline
Mood: No Mood
|
|
Wouldn't ammonia as a leaving group involve the ring breaking?
Unless that is a free chlorammonium has been formed, but for that to happen it'd also be necessary to have the ring break?
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
Quote: Originally posted by Sniffity  | | Other things I've read:Isocyanurate ions are actually hydrolized to form HOCl, is this true? Can't seem to find any basis for this?
|
Chloroisocyanurates are hydrolyzed to HOCl, yes.
| Quote: | | Perhaps the oxygen is protonated, and then the positive charge is passed over to the nitrogen via resonance? This just seems a little odd: Shouldn't
the product after the reaction with HCl be basically trichloroisocyanuric acid with all the chlorines replaced with Hs? |
You're on the right track. And the dechlorinated compound you're referring to is cyanuric acid. It exists as a pair of tautomers, one where the
nitrogens are protonated and one where the oxygens are protonated. Which tautomeric form dominates generally depends on pH.
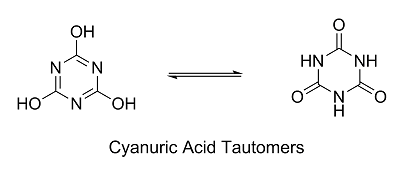
| Quote: | | I was thinking of the N-Cl bond: Is it actually shifted to the N side? I mean: Shouldn't the chlorine pull the electrons stronger than the nitrogen,
thus shifting the bond to the chlorine? (Then again: Nitrogen does have a lone pair, which could be vulnerable to protonotion).
|
It's not as simple as chlorine being more electronegative than nitrogen. There are both inductive as well as delocalization effects at play here.
| Quote: | | I can see the chloroamine's nitrogen being protonated. But what follows? I mean:Option 1: Something clearly has to leave that nitrogen, and it could
either be Cl+ or H+. My way of seeing it is the H+ will leave, given that Cl+ is not a good leaving group. |
First of all, I highly doubt TCCA's nitrogens are what get protonated under acidic conditions. If anything's going to get protonated, it's going to be
the carbonyl oxygens. The reason is due to resonance stabilization. If an oxygen gets protonated, the charge can be stabilized through electron
delocalization. If a nitrogen gets protonated, there's no way to stabilize the charge, making protonation at a nitrogen a lot less favorable. I made
the following to show you what I mean:
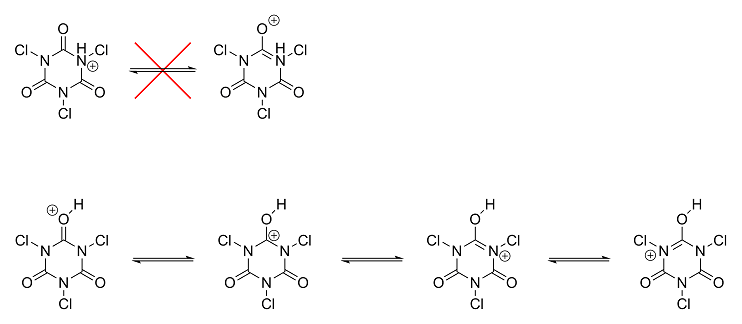
As you can see, with the top, the charge is more or less completely localized on the one nitrogen atom. With the bottom, however, the charge gets
delocalized and spread out across four different atoms.
Secondly, I doubt that a chloride ion directly attacks the protonated TCCA to form Cl2. According to the literature I've read in the past,
the general consensus seems to be that, under acidic conditions, TCCA is first hydrolysized to HOCl, even in the presence of excess chloride ions. (I
may attach a reference later to back this up. I'll have to get it off my phone) It seems that it's actually the hypochlorous acid that is the source
of the Cl2, not the protonated TCCA. Thus the chlorine gas is a result of the usual reaction between hypochlorous acid and HCl:
HClO + HCl ⇌ Cl2 + H2O
And as far as what byko3y said about TCCA hydrolyzing slowly in water, keep in mind that the hydrolysis reaction is reversible. This
means TCCA is in equilibrium with HOCl. So while the hydrolysis may be slow under normal conditions, under highly acidic conditions with excess
chloride ions the hydrolysis becomes extremely rapid due to Cl2 production. (can also be backed up with a reference if necessary) The
reaction with HCl that produces chlorine gas continuously lowers the concentration of HOCl, which, in turn, shifts the equilibrium over to the right
to favor more HOCl production via hydrolysis:
TCCA + 3 H2O ⇌ Cyanuric acid + 3 HOCl
HClO + HCl ⇌ Cl2 + H2O
Anyway, here's the general idea behind my proposed mechanism. Like most reactions involving chlorine and its various oxidative species, the exact
mechanism for the reaction between HOCl and HCl isn't known, either. But I imagine that it proceeds through either an activated hypochloronium ion via
nucleophilic attack by chloride, or though some kind of autocatalytic mechanism involving chloride, hypochloronium and water.
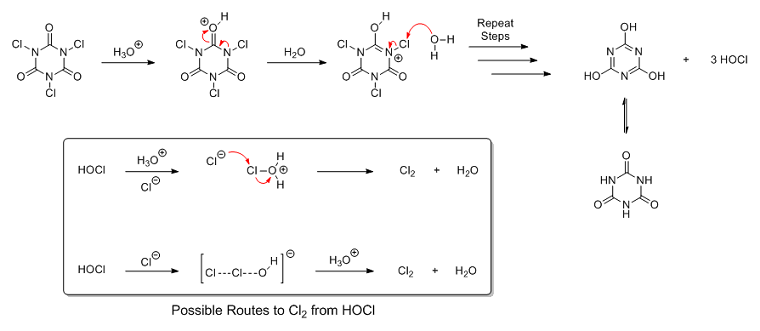
Here's a much higher quality (and resolution) version of the mechanism in .png format if you're interested:
|
|
|
Sniffity
Hazard to Self
 
Posts: 70
Registered: 27-12-2014
Member Is Offline
Mood: No Mood
|
|
Magnificent; exactly what I was looking for!!!   
Yeah, something did seem off about a chloride attack on TCCA itself; but by forming HOCl it all makes sense.
Thanks again!!!
Ps: I U2U'd you.. If you've got time give it a read 
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
I found myself wondering what would happen if I added hydrobromic acid to TCCA. Based on the above mechanism, I would expect an immediate product of
bromine monochloride. This would be unstable in the aqueous environment. The reaction seems to be:
1. BrCl + H2O -> BrOH + Cl(-) + H(+)
suggesting that hydrolysis might be slowed by strongly acidic conditions. (This would seem to support the pH dependence of the rate of the reaction:
http://www.ncbi.nlm.nih.gov/pubmed/11347924 )
I tried this today with a knifetip of TCCA and a few drops of hydrobromic acid I recently made. The solution darkened from yellow to orange to brown,
and there appeared a reddish brown gas, smelling very much like bromine, as well as a few insoluble red-black droplets.
I suspect that even near pH 0, hydrolysis causes a runaway bromine generation. In one pathway, hypobromite generated by the hydrolysis can react with
bromine monochloride, producing elemental bromine and hypochlorite
2a. BrCl + BrOH -> Br2 + ClOH
In another, hydrochloric acid generated by the hydrolysis reacts with TCCA or hypochlorite produced in 2a, forming elemental chlorine. This displaces
bromine from the bromine chloride and the hydrobromic acid, reforming chlorine and hydrochloric acid
2b. BrCl + HBr + Cl2 -> Br2 + HCl + Cl2
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
Sniffity
Hazard to Self
 
Posts: 70
Registered: 27-12-2014
Member Is Offline
Mood: No Mood
|
|
@Mayko
Interesting!! So based on the mechanism above, water + TCCA = HOCl. I guess we're delving into the land of hypochlorites, and all the strange things
that happen in the equilibrium of hypochlorite in water. If I'm not mistaken, there should also be a slight amount of chlorine gas and hydrochloric
acid present whenever there's hypochlorite dissolved in water..
Now, based on your proposal... the question comes to be.. If we were to add hydroiodic acid to the solution... We should get iodine monochloride
which, being unstable, should also degrade into elemental iodine, following a similar mechanism to the one you proposed..
Anyone with some spare HI and TCCA willing to test this out?
|
|
|
|