Electra
Hazard to Others
  
Posts: 179
Registered: 11-12-2013
Member Is Offline
Mood: No Mood
|
|
CanI store 68% nitric acid safely in HDPE bottles?
I've read the 99% nitric acid can break plastic caps, but what about normal 68%?
|
|
|
Detonationology
Hazard to Others
  
Posts: 362
Registered: 5-5-2015
Location: Deep South
Member Is Offline
Mood: Electrophillic
|
|
Glass is the preferred method for long term storage. Here is a thread about HNO3 stored in HDPE
“There are no differences but differences of degree between different degrees of difference and no difference.” ― William James
|
|
|
ganger631
Hazard to Self
 
Posts: 53
Registered: 15-8-2014
Member Is Offline
Mood: Plata o plomo
|
|
I have a bottle of 67% nitric acid that i purchased a while back(Around 1 year ago). I recently opened it and found out the cap was
degrading(nitrogen fumes expelling as well), it was not made of teflon. That said, to answer your question,i dont think nitric acid can be stored in
hdpe bottle(long term wise). i think you should invest is a heavy duty media bottle. I purchased a few media bottles from eBay, hopefully they'll do
the job. Good luck 
|
|
|
Dr.Bob
International Hazard
    
Posts: 2736
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
I would not store any solution of nitric acid (above maybe 1%) in plastic. It is an oxidizing acid, and thus will degrade plastic over time, maybe
even quickly. You are asking for a disaster. But I would store glass bottles inside of a rubber or plastic container as secondary containment.
And be aware that any trace of impurities in nitric acid can cause pressure buildups, so I might leave the cap ever so slightly loose, to avoid
pressure building up in the bottle, I have seen an example of a glass bottle breaking due to that when someone contaminated the bottle.
Sulfuric is not quite as dangerous at room temperature, but I would also store that in glass. HCl solutions of 2M and less appear stable in PE/PP
bottles, and are shipped that way.
|
|
|
Deathunter88
National Hazard
   
Posts: 519
Registered: 20-2-2015
Location: Beijing, China
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Dr.Bob  | I would not store any solution of nitric acid (above maybe 1%) in plastic. It is an oxidizing acid, and thus will degrade plastic over time, maybe
even quickly. You are asking for a disaster. But I would store glass bottles inside of a rubber or plastic container as secondary containment.
And be aware that any trace of impurities in nitric acid can cause pressure buildups, so I might leave the cap ever so slightly loose, to avoid
pressure building up in the bottle, I have seen an example of a glass bottle breaking due to that when someone contaminated the bottle.
Sulfuric is not quite as dangerous at room temperature, but I would also store that in glass. HCl solutions of 2M and less appear stable in PE/PP
bottles, and are shipped that way. |
Nitric acid can be stored in HDPE as long as you keep an eye on the condition of the bottle and replace it once it gets old. My Nitric acid has been
in a HDPE bottle for around 2 years and the bottle is still as good as new. Likewise with conc. HCl and H2SO4.
Here is a photo of a chemical resistance chart. (I found it after I stored 500ml of 98% sulphuric acid in a PET soda bottle. On the kitchen table...)
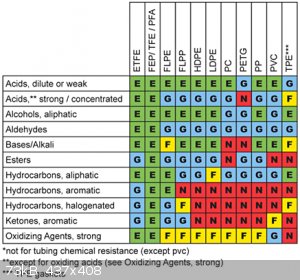 
[Edited on 6-10-2015 by Deathunter88]
|
|
|
xfusion44
Hazard to Others
  
Posts: 223
Registered: 6-8-2014
Location: Europe
Member Is Offline
Mood: Nostalgic
|
|
I'll go a little bit off topic here, but my experience with nitric acid and rubber stoppers might help someone when making decisions about proper
storage containers:
[Edited on 9-10-2015 by xfusion44]

|
|
|
ave369
Eastern European Lady of Mad Science
   
Posts: 596
Registered: 8-7-2015
Location: No Location
Member Is Offline
Mood: No Mood
|
|
My 90+% nitric acid does not appear to have an effect on polyethylene stoppers. But I'm storing it for weeks and months. Maybe, if stored for years,
it damages such stoppers.
Smells like ammonia....
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Again, if you have a need to Store acids in any volume for many years, then you have no actual Need for the acids.
People who Need them Use them.
|
|
|
xfusion44
Hazard to Others
  
Posts: 223
Registered: 6-8-2014
Location: Europe
Member Is Offline
Mood: Nostalgic
|
|
That erlenmeyer flask was almost full (I made that acid with KNO3+HCl+Cu method) and I used it for small experiments, but there was a little bit of it
left and I left it untouched for a few months...
|
|
|
xfusion44
Hazard to Others
  
Posts: 223
Registered: 6-8-2014
Location: Europe
Member Is Offline
Mood: Nostalgic
|
|
Quote: Originally posted by ave369  | | My 90+% nitric acid does not appear to have an effect on polyethylene stoppers. But I'm storing it for weeks and months. Maybe, if stored for years,
it damages such stoppers. |
The stopper you see on my picture is made of natural rubber (probably that's the reason why it was destroyed).
|
|
|
BobD1001
Hazard to Others
  
Posts: 182
Registered: 29-3-2013
Member Is Offline
Mood: No Mood
|
|
My cleanroom grade 70% nitric acid came in a 2L HDPE bottle with a Teflon lined cap. I have a glass safety coated amber bottle with a teflon lid ready
to go for it, but havent gotten around to transferring it. It has been in the HDPE bottle for over 2 years now with no signs of degradation. As a
precaution though, it is stored within two HDPE bags, which are then stored in a large HDPE bucked with a screw top lid.
|
|
|