morsagh
Hazard to Others
  
Posts: 187
Registered: 20-2-2014
Member Is Offline
Mood: No Mood
|
|
Maleic acid
How to make maleic acid from household chemicals? Can be used oxidation of benzoic acid by KMnO4?
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
You can buy malic acid from wine making stores. Subliming this produces maleic anhydride which you can add to water.
|
|
|
reactofurnace
Hazard to Self
 
Posts: 76
Registered: 17-7-2015
Member Is Offline
Mood: Volatile
|
|
you can oxidise benzene to make it or hydrolyse malic anhydride
|
|
|
Volanschemia
Hazard to Others
  
Posts: 340
Registered: 16-1-2015
Location: Victoria, Australia
Member Is Offline
Mood: Pretty much all of them!
|
|
The OP is asking about Maleic Acid (C4H4O4), not Malic Acid (C4H6O5).
You can make Maleic Acid via oxidation of Benzene I believe. If you don't have access to Benzene, you can make some by reacting Sodium Benzoate or
Benzoic Acid with Sodium Hydroxide and distilling off the Benzene. Nile Red has a video of the process here.
"The chemists are a strange class of mortals, impelled by an almost insane impulse to seek their pleasures amid smoke and
vapor, soot and flame, poisons and poverty; yet among all these evils I seem to live so sweetly that may I die if I were to change places with the
Persian king" - Johann Joachim Becher, 1635 to 1682.
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
Heating malic acid produces maleic anhydride (easily sublimes) and fumeric acid (which cannot form an intramolecular anhydride) which is left as a
mass of fibres. Stronger heating of the remaining fumeric acid isomerises and dehydrates it to more maleic anhydride. My memory of doing the first
stage of the reaction is that I just had to heat the molten malic acid and the crystals formed on a tin lid while the melt grew a mat of long fine
crystals with a much higher melting point. I wanted both products but didn't purify either.
This is just dehydration of an alcohol group to form a double bond. If the groups around are left as cis, that's maleic acid, trans and it's fumeric
acid. These reactions are normally not this easy but with malic acid they just work, mild conditions, no catalyst, no dehydrating agent. Yield if
taken all the way is over 50% from what I remember, possibly much higher.
Making maleic acid from benzene or benzoic acid or butane by the industrial route does not sound feasible at home to me and I would also expect the
oxidation by permanganate route to fail.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Malic, Maleic, and Fumaric acids
This report describes my failed preparation of maleic anhydride.
I used the Vogel (ref 1) preparation at ½ scale. The following equation is representative of what actually occurred:
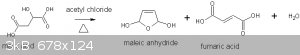
reaction equation
The HCl and CH3COOH by-products are not shown.
Reaction
22.5g of malic acid (note 1) was loaded into a 100mL RBF situated in a water bath on a magnetic stirrer. A Claisen head was affixed to the RBF. A
pressure-equalization (p-e) addition funnel and reflux condenser were attached to the Claisen adapter. The outlet of the condenser was led to an
inverted funnel over water for the absorbtion of HCl gas. 32g of acetyl chloride was added to the p-e funnel.

Reaction set-up
The acetyl chloride was added slowly with stirring. The reactants were actively bubbling and refluxing. Heating/refluxing was continued for 1.5hrs
after the bubbling had stopped.
Distillation
The product mix was then reconfigured for standard downward distillation to recover the product as maleic anhydride which has a bp of 202°C. ~20mL
of light boiling material came over at about 123°C. The receiver was then changed to collect the maleic anhydride. I’m guessing that about 5g of
product was collected in a 125mL Erlenmeyer flask as shown below:

products from malic acid
As can be seen there was a great deal of white crystals in the pot (~ 10g in the beaker). This is where I made a big mistake. I assumed that this
was maleic anhydride that had not distilled over (wrong!). So I mixed the two products together.
Solvent Extraction
I then attempted to extract the maleic anhydride with chloroform per Vogel. But there was no maleic anhydride. The product had all been converted to
fumaric acid, the geometric isomer of maleic acid.
After dissolving the fumaric acid in hot water the solution was filtered to remove bits of char. The filtrate was then evaporated to remove excess
water.
Results
The fumaric acid was then crystallized to 6.7g of easily filtered, slightly off-white crystals. A sealed-tube melting point determination indicated
296°C (lit value: 287°C).
Discussion
Fumaric acid is the trans isomer of maleic acid, the cis isomer. Fumaric acid is the more stable isomer, having a lower energy of
formation. I have done an ACS journal search to find a method of converting the fumaric acid to maleic acid but could find none. Louis and
Mary Fieser in their textbook (ref 2) mention that fumaric acid can be converted to maleic acid at 75% conversion by treatment of fumaric acid with
uv light, but no reference is provided. I have also read that fumaric acid can be converted to maleic anhydride by dehydration.
I will do some limited experimentation with P4O10 to attempt dehydration. I might also try uv light.
Note 1: Malic acid can be purchased cheaply OTC as a brewcraft supply.
References
1. “A Textbook of Practical Organic Chemistry,” 3rd ed, (1956), by A.I. Vogel, p. 462.
2. “Introduction to Organic Chemistry,”(1957), by Louis & Mary Fieser, p. 224.
Your comments, questions, and recommendations are encouraged.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
AvBaeyer
National Hazard
   
Posts: 651
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
Magpie,
Fumaric acid is pretty much a "dead end" product. There is no reasonable way to convert it to maleic acid.
Now, in the reaction you ran I suspect that the dehydration was fairly rapid but due to the high temperature you were using there was rapid
isomerization of any maleic acid to fumaric acid which in turn was faster than anhydride formation. Note that maleic acid is easily isomerized by HCl.
I believe that this is documented in Vogel as a preparative experiment. I believe that the "one pot" conversion of malic acid to maleic acid is best
done with a low boiling dehydrating agent like trifluoroacetic anhydride. By keeping the temperature down, isomerization is minimized.
Interestingly, the anhydride of O-acetylmalic acid is known and easily prepared. This may be convertible in a second step to maleic anhydride. I am
out of the country for a while and not able to give a reference for this but I am pretty sure I have one in my files. However, you should be able to
uncover this in a literature search.
Hope this is useful,
AvB
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
If you really needed to, you could hydrate the fumaric acid back to malic acid (like the step in the Krebs cycle), and try again.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Thanks for the advice saving me effort and P2O5.
Malic acid us cheap and starting over sounds like the best move. However, this means I have to make some more acetyl chloride or buy the reagent you
mention, or buy maleic acid. The last option being cheapest but the least satisfying.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
Heat alone will turn fumeric acid into maleic anhydride. Phosphorus pentoxide would make the process much easier but it may be overkill. You could
try metaphosphoric acid instead, which pretty much can't fail and would be much easier to do.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I took 8g of my recently made maleic anhydride and converted it to maleic acid by hydrating it using the procedure in Vogel's 3rd ed, p.462.
Per procedure I added 4ml of water to the anhydride in an evaporating dish and set it floating on a water bath as shown:

This was heated for sometime (hours) but never looked completely dry and still smelled of acetic acid. I then placed the dish in a dessicator over
con H2SO4 and left overnight. It looked much better the next day but still not dry and still smelling. I then took a melting point and found it to
be 127°C with the Wiki value being 135°C. The product is shown here:

Then, per procedure, I recrystallized the product in acetone-pet ether. The resulting crystals looked dry and did not smell. The melting point was
137°. Incidentally this is the same mp that I obtained on some commercial maleic acid that I happened to have had on hand. Here's a poor picture of
the final product:

Results Yield was 6.1g, or 64%. Basically all the loss occurred during the recrystallization from acetone-pet ether.
Discussion My previous fumbling attempt to make this acid had resulted in making fumaric acid instead. I was very pleased this
time when my impure product mp was 127°C as this indicated that I had impure maleic acid instead of fumaric acid (mp 287°C), which is the
thermodynamically more stable isomer.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|