| Pages:
1
2 |
Romix
Hazard to Others (Literally)
  
Posts: 483
Registered: 19-6-2015
Member Is Offline
Mood: No Mood
|
|
Copper Citrate (and other transition metal citrates)
Pure copper anode and cathode, electrolysed through sodium carbonate.
Blue hydroxide formed on anode. Had to change polarity every 10 minuts, cos it sticks to electrode, and blocks conductivity.
This hydroxide I dissolved in citric acid, produced alot of bubles. I got told that what I call hydroxide is basic copper carbonate. And bubles
produced are carbon dioxide.
That's how I balanced equation for this reaction 2C6H8O7 + 3Cu(OH)2 = Cu3(C 6H5O7)2 + 6H2O
There should'nt be any bubles.
[Edited on 1-7-2015 by Romix]
[Edited on 1-7-2015 by Romix]
[Edited on 1-7-2015 by Romix]
Edit by Texium: changed title with thread merge, for clarity
[Edited on 9-14-2023 by Texium]
|
|
|
papaya
National Hazard
   
Posts: 615
Registered: 4-4-2013
Member Is Offline
Mood: reactive
|
|
You produced malachite, that's why it's bubbling, however the exact chemical structure of that deposit might be different and cannot be reliably
identified (how much hydroxide vs basic carbonate is formed, how hydrated is it, OH- anions sometimes serve as ligands for transition metals,
sometimes bridge type ligand, etc,etc..). For such type of "compounds" I have one description only - sludge!
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by papaya  | | You produced malachite, that's why it's bubbling, however the exact chemical structure of that deposit might be different and cannot be reliably
identified (how much hydroxide vs basic carbonate is formed, how hydrated is it, OH- anions sometimes serve as ligands for transition metals,
sometimes bridge type ligand, etc,etc..). For such type of "compounds" I have one description only - sludge! |
No, no. What you write here is factually very incorrect. The composition of Malachite is well defined: equimolar Cu(OH)2 + CuCO3 = Cu2CO3(OH)2.
https://en.wikipedia.org/wiki/Malachite: scroll down to half way down for a ball and stick model.
There's NO reason to believe that electrolytic basic copper carbonate would have a different structure or have a different hydroxide/carbonate ratio.
Water isn't chemically bound to it, so that's different.
In Romix's process, Cu<sup>2+</sup> is formed through electrolytic oxidation. Then:
2 Cu<sup>2+</sup>(aq) + 2 CO<sub>3</sub><sup>2-</sup>(aq) + H<sub>2</sub>O(l) === >
Cu<sub>2</sub>CO<sub>3</sub>(OH)<sub>2</sub>(s) + CO<sub>2</sub>(g)
That compound is definitely NOT 'sludge'! 
[Edited on 1-7-2015 by blogfast25]
|
|
|
papaya
National Hazard
   
Posts: 615
Registered: 4-4-2013
Member Is Offline
Mood: reactive
|
|
I agree that the malachite is well defined, but it's still possible that under not very well controlled conditions what will form is not only
malachite. Look at your equation
2 Cu2+(aq) + 2 CO32-(aq) + H2O(l) === > Cu2CO3(OH)2(s) + CO2(g)
The release of CO2 means that if you use small volume of carbonate solution it will probably lower the pH over the time and go more to bicarbonate
side, what happens then I don't know. But if you have information that this process is really reliable for malachite production - I cannot object.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by papaya  |
The release of CO2 means that if you use small volume of carbonate solution it will probably lower the pH over the time and go more to bicarbonate
side, what happens then I don't know. But if you have information that this process is really reliable for malachite production - I cannot object.
|
Sigh... Malachite is formed BOTH by carbonate AND bicarbonate solutions. Only pure hydroxide forms Cu(OH)2.
2 Cu<sup>2+</sup> + 4 HCO<sub>3</sub><sup>-</sup> === >
Cu<sub>2</sub>CO<sub>3</sub>(OH)<sub>2</sub> + 3 CO<sub>2</sub> + H<sub>2</sub>O
So with bicarbonate you're simply wasting more CO2.
The only way you might run into Cu(OH)2 with Romix's process is by running out of carbonate or bicarbonate and then you're simply making malachite
first, then copper hydroxide.
There's an awful lot of misinformation on the web re. copper carbonate, carbonates and bicarbonate, spread by amateurs that make salient claims
without ever having measured a g-ddamn thing!
Also, released CO2 cannot lower the pH of a carbonate solution. Small or large volume has nothing to do with anything: we're talking concentration
here.
[Edited on 1-7-2015 by blogfast25]
|
|
|
Romix
Hazard to Others (Literally)
  
Posts: 483
Registered: 19-6-2015
Member Is Offline
Mood: No Mood
|
|
I don't understand how basic copper carbonate formed.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
If you can't understand the explanation offered above then I can't help you, sorry.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
I also think you prepared dicopper citrate, not tricopper citrate (as you claim). I could be wrong on that.
[Edited on 2-7-2015 by blogfast25]
|
|
|
violet sin
International Hazard
    
Posts: 1482
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
Romix: here ya go, if you don't see where the hydroxide ion is coming from, to quote blogfast25:
----------
n Romix's process, Cu<sup>2+</sup>is formed through electrolytic oxidation. Then:
2 Cu<sup>2+</sup>(aq) + 2 CO3<sup>2-</sup>(aq) + H2O(l) === >
Cu2CO3(OH)2(s) + CO2(g)
----------
one of the CO3<sup>2-</sup>(aq) has to release an oxygen to become CO2(g) , and water H2O(l) gains that
to become 2 OH<sup>-</sup>(aq)
CO3<sup>2-</sup>(aq) + H2O(l) -> CO2(g) + 2 OH<sup>-</sup>(aq)
the charge is maintained, but i'm a bit rusty on explaining *exactly how/why/where that happens,
I have also heard people say (on youtube so grain of salt here) that the Na<sup>+</sup>(aq) participates by traveling to the electrode,
receiving an e<sup>-</sup> -> Na<sup>0</sup> and instantaneously 2 [ Na<sup>0</sup> + H2O(l) ] ->
2 Na<sup>+</sup>(aq) + 2 OH<sup>-</sup>(aq) + H2(g)
^^^( open for criticism)^^^
man, it is a bit tedious to do all the sub/sup by hand...
|
|
|
Romix
Hazard to Others (Literally)
  
Posts: 483
Registered: 19-6-2015
Member Is Offline
Mood: No Mood
|
|
Oxygen on anode, hydrogen on cathode, copper oxidized to Cu + 2 , not going far drops to the bottom of electrode, and stick to it after a while
blocking conductivity.
[Edited on 2-7-2015 by Romix]
|
|
|
violet sin
International Hazard
    
Posts: 1482
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
https://en.m.wikipedia.org/wiki/Basic_copper_carbonate
Basic copper carbonate is insoluble,.. so If you have hydroxide and carbonate anions roaming free, and they happen across a swarm of copper
cations... Theyz get married and settle down.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
This is kind of the key point: Cu2CO3(OH)2 is more insoluble than Cu(OH)2 or CuCO3, that's why it precipitates preferentially, if
carbonate/bicarbonate is present.
|
|
|
sasan
Hazard to Self
 
Posts: 92
Registered: 22-2-2014
Location: TEHRAN / IRAN
Member Is Offline
Mood: Radiative
|
|
Hi,there is another method using sodium citrate and copper sulfate with 1 : 1.5 molar ratio.make the solutions and treat with each other.leave it for
a night and filter the precipitate.
http://www.rsc.org/learn-chemistry/resource/download/res0000...
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Yep.
I'm not convinced that citric acid is strong enough to fully neutralise basic copper carbonate.
The third deprotonation of citric acid (H<sub>3</sub>Ct) has a pK<sub>a3</sub> of only 6.4, that's extremely weak and copper
basic carbonate is NOT a strong alkali.
To be clear, the third dissociation:
HCt<sup>2-</sup> + H<sub>2</sub>O <=== > H<sub>3</sub>O<sup>+</sup> +
Ct<sup>3-</sup>
... is extremely weak.
Did Romix actually obtain a precipitate?
[Edited on 2-7-2015 by blogfast25]
|
|
|
papaya
National Hazard
   
Posts: 615
Registered: 4-4-2013
Member Is Offline
Mood: reactive
|
|
Citric acid is a very strong complexant, it doesn't need to be a strong acid to dissolve carbonate, but of course you know it blogfast!
|
|
|
violet sin
International Hazard
    
Posts: 1482
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
tonight I checked the (supposed) copper hydroxide I made a few years back, still light sky blue in it's mason jar after all this time. it did not
bubble the least with strong HCl( picture below just moments after addition). pool/concrete etchant strength from the hardware store. I got the how
to from youtube as copper electrodes in a dilute(ish) aqueous epsom salt bath, with a hacked ATX computer PSU. the sample instantly turned a
beautiful clear emerald green when the HCl was poured in, dissolving the clumps rather fast.
 
after I cleaned the test tube out, pleased that it didn't fizz, I also put some of the copper hydroxide in an iron citrate solution I had just made. [
rust and a little steel wool in excess citric acid, all dissolved; made a clear darker yellow sol pictured below, top two]. the gloppy blue copper
hydroxide paste( I had kept it wet) was shook in a few ml of the iron citrate. after the cloudiness settled out in a few min, the solution began
turning rather green( pictured below, bottom 2). I honestly don't know how much free citric acid was left in the iron citrate sol, or how much rust
and steel wool I had in there. I was just hoping to make some crystals later.
so, no I didn't make copper citrate directly, but I did test the copper hydroxide I made(with HCl) and then get the iron citrate I had already made,
to take up some copper. not a bubble in either case.
 .................................................... .................................................... 
 .................................................... .................................................... 
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by papaya  | | Citric acid is a very strong complexant, it doesn't need to be a strong acid to dissolve carbonate, but of course you know it blogfast!
|
I hadn't thought of that, papaya, no. Ta. 
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Violet sin:
The Fe citrate complex (FeCt) is likely stronger than Cu3Ct2. Thus no ligand transfer occurs:
FeCt + CuCl2 === > Fe chloride + Cu citrate doesn't happen.
Try neutralising some NaOH with citric acid, then add this to your CuCl2 solution, that's more or less what 'sasan' linked to above.
Na<sup>+</sup> cannot be complexed by citrate ions, so you have free Ct<sup>3-</sup> anions in there.
[Edited on 3-7-2015 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
For the benefit of ‘violet sin’ (his request via U2U):
The equilibria that rule a solution that contains a dissociated copper(II) salt a soluble carbonate are:
1) Re-protonation of carbonate anions:
CO3(2-) + H2O < ==== > HCO3- + OH-
Leans much to the left but causes the solution to be alkaline.
2) Auto-dissociation of water:
2 H2O < === > H3O+ + OH-
Leans very much to the left.
3) Re-protonation of bicarbonate anions:
HCO3- + H3O+ === > H2CO3
Leans much to the right.
4) Decomposition of carbonic acid:
H2CO3 < === > H2O + CO2(g)
Leans much to the right.
5) Solubility product of basic copper carbonate:
Cu2CO3(OH)2 < ==== > 2 Cu2+ + CO3(2-) + 2 OH-
Leans much to the left.
One can set up the relevant equilibrium equations (including equilibrium constants, all are known), including mass balance and neutrality balance and
solve this system of simultaneous non-linear equations for the concentrations of all species and would conclude that:
2 Cu2+ + 2 CO3(2-) + H2O === > Cu2CO3(OH)2(s) + CO2(g)
… proceeds as observed.
More simply put, the formation of an insoluble reaction product and a gaseous one, ensures that the change in Gibbs Free Energy ΔG (left to right for
the overall reaction) is negative, i.e. favourable.
|
|
|
violet sin
International Hazard
    
Posts: 1482
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
-The mix was copper hydroxide, iron citrate with an excess of citric acid. And it turned green. Either the excess citric acid in solution dissolved
more of the Cu(OH)2 overnight, or it settled more compactly. I'll add some more citric acid to see if it will eat all the hydroxide. Oh
ya, there is no brown ppt in there. Indicating the copper hydroxide didn't displace the iron, both are in sol.
-I had the iron citrate sitting around b/c I wanted some crystals of just that. It wasn't purpose made for this experiment, just convienient.
-The copper chloride was dumped in my copper chloride e-waste jug. And did not participate here at all. Just for the carbonate test.
----------
all the copper hydroxide dissolved leaving a clear green solution.
[Edited on 4-7-2015 by violet sin]
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
I have prepare Copper citrate fairly quickly by simply combining Copper metal, Citrate acid, dilute H2O2 and a touch of sea salt (or plain salt) to
act as a good electrolyte for the galvanic cell reaction (think/research metal-air battery where the H2O2 replaces O2, and with an acidic electrolyte,
the strength of the conjugate base, dihydrogencitrate, is involved).
I usually jump start the reaction by heating the mixture in a microwave, at which point, the reaction actually appears to be quite vigorous. In
minutes, my experience is that the solution looks like the above pictures.
No external current or electrodes required or even continuous heating, for that matter. You may have to add more H2O2 and reheat periodically until
the Citrate acid, is completely consumed, however.
Note, the final product will have some NaCl impurity, which if is an issue for an intended application, could be addressed through recrystallization.
Also, ones copper source may be an alloy, resulting in yet another source of possible impurities. Even very high purity electrical Copper wiring, for
example, may contain some Cadium (see discussion at http://www.copper.org/resources/properties/microstructure/ca... ).
[Edited on 5-7-2015 by AJKOER]
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
Here are some notes from my explorations of copper citrate. The preparation came from the RSC document in the Sources section:
Experimental and Observations
32.11 g (0.157 mol; 1 eq) citric acid monohydrate was added to a 250 mL beaker containing 150 mL distilled water, and the mixture was heated to
dissolve. The pH of the solution, measured with multi-indicator test strips, was ~1.5. 22 g of sodium hydroxide were added (0.55 mol, or 3.7 eq,
assuming 0% moisture in the hydroxide), bringing the pH to ~12. The liquid was poured into an evaporating dish and heated until bubbling gently and
slightly syrupy. As it cooled, the liquid began to form a sort of 'pudding skin' on the surface. In the unheated lab, the mixture froze solid
overnight; on warming, it remained a solid cake of white crystalline solid. This was broken up and added to a 250 mL beaker containing 50 mL of 200
proof ethanol. The slush was stirred, filtered and rinsed with another 50 mL dry ethanol. Once the solid had drained thoroughly, it was crushed finely
and stirred into 100 mL boiling dry ethanol. After several minutes, it was filtered and laid out to dry. The white, fluffy crystalline solid massed at
46.24 g (0.157 mol; 1 eq; i.e., nearly qualitative yield.) A solution of the salt was measured at pH~10, compared to a literature value of 8,
suggesting residual hydroxide contamination. Adding copper sulfate to the solution did not yield precipitate; the mixture appeared to darken with
small dilution.
In an erlenmeyer flask, 5.91g sodium citrate (0.02 mol, 1eq) and 7.54g copper sulfate pentahydrate ( 0.03mol. 1 eq) were dissolved in steaming
distilled water, creating a definite darkening of the copper blue to a deep teal. On standing, a scummy deposit, not a grained precipitate, formed.
Upon heating, the solution precipitated a fine turquoise powder. This was washed with alcohol, dried, and massed at 3.39 g . Taking at face value the
hydration state in Merck of 2.5 and the hydrate molar weight of 613 g/mol, this is ~0.0055 mol, ie 55% yield. (There were mechanical losses when the
flask burped in the microwave.)

To confirm the hydration state, 0.50 g copper citrate were placed in a test tube and heated in a 50 mL beaker containing mineral oil (dehydration
occurs at 100C, says Merck). Moisture was seen leaving the tube, and the solid changed from a sea-foam green to a sky blue. The pale dehydrated salt
was massed at 0.46 g (0.81mmol). This suggests 0.04g water (2.2mmol) were driven off, giving a ratio of ~2.75 water molecules per copper citrate
molecule, agreeing with the literature value.
 
A small amount was placed in a test tube and heated while continuously flushed with carbon dioxide. Granular metallic copper resulted, though no
pyrophoricity was observed.

RSC provides an alternative procedure, based upon copper acetate and citric acid, rather than copper sulfate and trisodium citrate. In a 50 mL flask,
1.50g copper acetate (8.2mmol) and 1.09g (5.1mmol) were dissolved in ~20 mL distilled water. On standing, a small amount of green crust formed. When
the liquid was poured into a new flask and heated to boiling, it again became turbid green and precipitated. (Below, left) Magnesium citrate,
available in solution at the pharmacy as a laxative, will also precipitate when heated with copper sulfate. (Below, right)
 
Merck claims that copper citrate is soluble in hot solutions of alkali citrates; this was attempted with hopes of recrystallization. In 25 mL of
boiling distilled water were dissolved 3.4 g trisodium citrate, and 1.6 g copper citrate was added. The mixture was at first turbid and murky, then
became a dark, clear blue solution. This was filtered hot into a new 50 mL flask cooled to room temperature, then chilled. After two days, no crystals
were observed; the flask was heated down to ~5mL to drive off water. This was chilled another day, but only became more syrupy. When a drop was placed
in a test tube of pure water, or a 50/50 mixture of water and ethanol, the liquid became a clear blue solution; in 100% ethanol, the drop settled on
the bottom like a bead and stuck to the glass. When ~1mL 100% ethanol was added to the flask containing the syrup, it formed a bilayer (!) which
would reappear even when mixed with vigorous shaking (!!). Over several months, the liquid solidified into a pale blue crust. The solubility of copper
citrate in concentrated ammonia was also confirmed, forming a deep tetraamine-copper blue solution.
  
Data & Materials
Citric acid C6H8O7 (mw=192.12 g/mol). Exists as a monohydrate (mw = 210.14g/mol); LD Carlson Co. product was used, via 5th Season, a local homebrew store.
Trisodium citrate Na3C6H5O7 (mw = 258.07 g/mol) Exists as a dihydrate (mw = 294.1 g/mol). Soluble in water, insoluble in alcohol
Copper (II) Citrate Cu3(C6H5O7)2 ; Cu3C12H10O14. mw = 568.85 g/mol . Hydration state claimed by Merck is 2.5 (mw = 613 g/mol)
Sodium Hydroxide NaOH (mw = 40g/mol) Comstar “99% Pure Lye“ was used. Slightly soluble in alcohol.
Copper acetate (mw=181.6 g/mol)
Copper sulfate pentahydrate. (mw = 250 g/mol)
Magnesium Citrate. Store-brand lemon-flavored laxative solution was used.
Discussion
This project grows out of an interest in pyrophoric copper, which can be prepared by heating in vacuum according to Gorrie, Kopf, & Toby (1967)
(h/t ScienceMadness user Pok). Lacking a vacuum pump, this could not be replicated using a CO2 atmosphere, although metallic copper did appear to
result from thermal decomposition. Although the original purpose of this investigation (pyrophoric copper) was not achieved, several interesting
observations were made. Some of these suggest directions for future exploration.
The first is misinformation and its propagation in the literature. Neither protocol given in RSC would work satisfactorily without additional heating.
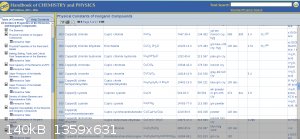
More severe is an error present in both the Merck Index [8th ed] and the CRC [63rd ed], listing the formula for copper (II) citrate as
“Cu2C6H4O7*2.5H2O”, with a molar weight of “63.220”. Although casual consideration of the citric acid acid molecule suggests this is unlikely
(it would require the deprotonation of a hydroxyl group in addition to three carboxylic acid groups), this formula has spread to internet databases and peer-reviewed literature The CRC still lists the incorrect formula at the time of writing [96th Ed, 2015-2016]

When prepared, the sodium citrate seemed to thicken and gel, rather than crystallize, then solidified into a block. When copper citrate was dissolved
in a sodium citrate solution and evaporated, it formed a syrupy fluid which slowly solidified. Perhaps in both cases, the salt(s) dissolved in the
water of hydration upon heating. Could this explain the behavior of the copper solution, if the salts were dissolved in too much water to form an
anhydrous solid, but too little to form the proper hydrate, forcing it into a fluid state until sufficient moisture could be absorbed from the
atmosphere? I have heard similar reports from attempted crystallizations of laxative magnesium citrate solution.
The darkening of the reaction mixture in spite of a dilution of the copper ion, would be an interesting area for spectrophotometric investigation.
References
Preparation of copper(II) citrate. RSC Student worksheet, http://www.rsc.org/learn-chemistry/resource/download/res0000...
CRC Handbook of Chemistry and Physics
Merck Index, 8th Edition
Gorrie, T., Kopf, P., & Toby, S. (1967). Kinetics of the reaction of some pyrophoric metals with oxygen. The Journal of Physical Chemistry,
1478(4), 3842–3845. Retrieved from http://pubs.acs.org/doi/pdf/10.1021/j100871a019
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
Quote: Originally posted by mayko  | Magnesium citrate, available in solution at the pharmacy as a laxative, will also precipitate when heated with copper sulfate.
|
I realized the other day that there are actually two distinct magnesium citrate salts:
*there is a less soluble trimagnesium citrate, with the formula Mg3Cit, and
*the sort that is used in this laxative, which is referred to as magnesium citrate but might be better called magnesium hydrogen citrate, MgHCit.
It occurred to me that I'd never confirmed that the copper salt I prepared from the laxative solution was the 3:2 salt, rather than a 1:1 hydrogen
salt like CuHCit.
To find out, I heated down 30 mL of the laxative (at ~1 fluid oz; at 1.745 g/flOz and 214 g/mol, that's ~0.008 mol) to drive off some water, and
dissolved ~3 g copper sulfate pentahydrate (approx. stoichiometric) in a minimum of water. a bit of each solution was touched to some universal pH
paper, with no color change except a bit of bluing from the copper sulfate. The two solutions were then mixed and heated until precipitation occurred.
The solution was checked again with indicator paper, which now turned orange-red.
If the product is the 3:2 salt, the citric acid must lose both a magnesium cation and a proton. If it's the 1:1 hydrogen salt, it will only add
magnesium to solution. The change in pH from approximate neutrality of the reactant solutions to quite acidic in the product supports the 3:2 salt.
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
I love how mayko swiftly ended all the bickering and conjecture above and nobody said anything about it. Mayko, thank you for such an exhaustive
report!
I've had two perplexing vials of both the anhydrous and hydrated forms of this copper citrate complex sitting on my lab windowsill for what seems like
an eternity that I inadvertently produced using a powdered drink mix... yeah. I was completely stumped at the time as to what they could've been and
before long I had forgotten how I obtained them in the first place, until seeing your pictures in the wiki reminded me of that afternoon where a
less-learned me poured lemon-flavored Kool-Aid powder into some copper salt solution in a half-witted attempt to make copper(I) oxide. Anyway, thanks
again!
[Edited on 12-12-2016 by Amos]
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
Quote: Originally posted by mayko  |
To confirm the hydration state, 0.50 g copper citrate were placed in a test tube and heated in a 50 mL beaker containing mineral oil (dehydration
occurs at 100C, says Merck). Moisture was seen leaving the tube, and the solid changed from a sea-foam green to a sky blue. The pale dehydrated salt
was massed at 0.46 g (0.81mmol). This suggests 0.04g water (2.2mmol) were driven off, giving a ratio of ~2.75 water molecules per copper citrate
molecule, agreeing with the literature value.
|
The 2.5 hydration multiplicity kept sticking out in my mind and I'm starting to doubt it. My earlier gravimetric measurement of the hydration state
was based on a relatively small mass (~half gram) with a low precision (0.01g) scale; additionally, the water molecules are much less massive than the
copper citrate (by a factor of ~30), meaning the difference in mass between the hydrated and dehydrated samples was miniscule (0.04g). Assuming this
difference is accurate to +/- 0.01g, this experiment could actually support a range of hydration multiplicities from 2 to 3.5.
I've done a few experiments with larger starting masses and they have generally indicated a multiplicity of 2. For example:
9.53g Cu3Cit2 * nH2O were heated to dehydrate the remaining Cu3Cit2 massed at 8.96 g (ie, 15.8 mmol). The difference of 0.57 g (ie, 31.6 mmol water)
gives a hydration multiplicity of n=2.01.
The main source of error, I'd expect, is from mechanical losses; these would tend to bias the calculated multiplicity upwards (ie, greater than 2.5).
Contamination or incomplete dehydration might biase the value downwards (ie, below 2.5) but I've come up with a value pretty close to 2 several times
now.
| Quote: |
This project grows out of an interest in pyrophoric copper, which can be prepared by heating in vacuum according to Gorrie, Kopf, & Toby (1967)
(h/t ScienceMadness user Pok). Lacking a vacuum pump, this could not be replicated using a CO2 atmosphere, although metallic copper did appear to
result from thermal decomposition. Although the original purpose of this investigation (pyrophoric copper) was not achieved,
|
This sort of depends on your definition of pyrophoricity; I still haven't observed the powder catch fire when poured out cold, but when poured
h | Quote: |
The solubility of copper citrate in concentrated ammonia was also confirmed, forming a deep tetraamine-copper blue solution.
|
I was able to isolate this as a dark blue solid two different ways. In the first, I would dissolve copper citrate in ammonia solution, and then add
dry alcohol (91%IPA), forming a bilayer similar to the one pictured above. This was mixed, left to stand, and the alcohol replaced. Eventually, the
complex formed a solid crust, which was further dried by grinding under dry ethanol:

This is problematic for a lot of reasons: much too lossy for gravimetry; takes a lot of alcohol; time consuming; raw product is quite tough and
difficult to dislodge. So, I tried another method, in which copper citrate was placed in a large test tube, covered in dry alcohol, and ammonia gas
bubbled through. The suspension becomes flocculent and darkens; on standing, a dark blue solid remains.

The experimental apparatus
 
The reaction mixture early (left) and late (right) in the process.

Top to bottom: Hydrated, dehydrated, and ammonia complex.
I've tried a few times to measure the multiplicity for the ammonia complex, and haven't been able to get much of anywhere. I think a number of factors
are working against gravimetry as a tool here, but it's not obvious what else is available.
Junk gravimetry results:
| Code: |
Copper Citrate, anhydrous equivalent Calculated amination multiplicity, given:
Copper Citrate mass (g), hydrated hydration multiplicity = 2 hydration multiplicity= 2.5 Aminated Copper Citrate hydration multiplicity = 2 hydration multiplicity= 2.5
1.05 0.99 0.97 0.9 -2.96 -2.5
1.01 0.95 0.94 0.98 1.06 1.57
5.01 4.71 4.64 4.64 -0.51 0
|
Anhydrous starting material:
| Code: |
Copper citrate mass, anh Aminated Copper Citrate calculated amination multiplicity
1.04 0.96 -0.58
|
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
| Pages:
1
2 |
|