Leo Szilard
Harmless

Posts: 20
Registered: 10-5-2015
Location: USA
Member Is Offline
Mood: No Mood
|
|
Diazonium Question
Good Day. Does anyone have any experience with diazotization? Specifically, I'm curious about the reaction of aryl diazonium salts with H3PO2. This
reaction seems super powerful because of the ability to direct additional substituents with an amino (or nitro) group; after which, you can "pluck
off" the amine. Are there any common alternatives to H3PO2? Book or lit references are greatly appreciated!
Additionally,
If there is a different discussion board in which I should post questions like this, let me know. I don't want to clog up this board unnecessarily.
(Picture from http://chemwiki.ucdavis.edu/Organic_Chemistry/Amines/Reactio...)
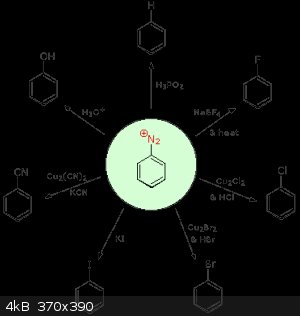
[Edited on 11-5-2015 by Leo Szilard]
|
|
|
mnick12
Hazard to Others
  
Posts: 404
Registered: 30-12-2009
Location: In the lab w/ Dr. Evil
Member Is Offline
Mood: devious
|
|
This should be in the beginings sections.
I just did a diazo reaction a few weeks ago with 2,6-diisopropylaniline, NaNO2/HCl, and KI. It was a simple enough reaction, but the work up was a
pain in the ass. A steam distillation and vacuum distillation have not adequately cleaned the product. So it looks like ill be distilling again, so at
this point im probably looking at like 40% yield.
I have not done it with H3PO2, what are you trying make?
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Diazonium salts react with hypophosphorus acid to replace the diazonium group with a hydrogen. Leo Szilard, to answer your question, basic sodium
stannite(the product of dissolution of tin metal in excess sodium hydroxide) will react in a similar fashion as long as your substrate isn't alkali
sensitive. If you would like, I can find some references for this reaction.
EDIT : Fixed typo
[Edited on 5-12-2015 by gdflp]
|
|
|
Nicodem
|
Thread Moved
11-5-2015 at 11:33 |
Leo Szilard
Harmless

Posts: 20
Registered: 10-5-2015
Location: USA
Member Is Offline
Mood: No Mood
|
|
mnick, I'm not trying to make anything in particular; rather, I just want to better understand the scope of the reaction. Thanks for your input.
|
|
|
Molecular Manipulations
Hazard to Others
  
Posts: 447
Registered: 17-12-2014
Location: The Garden of Eden
Member Is Offline
Mood: High on forbidden fruit
|
|
I made the diazonium salt of GABA (N2^+CH2CH2CH2COOH) in situ by addition of hydrochloric acid to sodium
nitrite and GABA (4- aminobutanoic acid). Since this was only an intermediate in my reaction to GBL (dyhydrofuran-2(3H)-one) and finally GHB
(4-hydroxybutanoic acid) I made no attempt to isolate it, however. I let it decompose for two days at room temperature and it was still evolving
nitrogen (in aqueous sol!) so I believe it is stable enough to isolate if done quickly enough.
For those interested, after it fully decomposed (by heating it) the produced GBL was extracted with dichloromethane and distilled (BP ~200C IIRC) and
then treated with sodium hydroxide to make the sodium salt of GHB.
[Edited on 12-5-2015 by Molecular Manipulations]
-The manipulator
We are all here on earth to help others; what on earth the others are here for I don't know. -W. H. Auden
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
Gdflp I'd like to see that because I've never heard of it.
Leo - I've heard that ethanol and heat can be used similarly. I have no experience with the reaction and as mnick12 stated sandmeyers tend to be
pretty dirty reactions.
|
|
|
Molecular Manipulations
Hazard to Others
  
Posts: 447
Registered: 17-12-2014
Location: The Garden of Eden
Member Is Offline
Mood: High on forbidden fruit
|
|
Arenediazonium salts certainly do react like gdflp said, although I'm not sure if straight chain diazonium salts do as he
implied by "diazonium salts". Don't see any reason for both not to work though.
See this Wikipedia page on diazonium salts.
[Edited on 12-5-2015 by Molecular Manipulations]
-The manipulator
We are all here on earth to help others; what on earth the others are here for I don't know. -W. H. Auden
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Picric-A makes a mention of that here : https://www.sciencemadness.org/whisper/viewthread.php?tid=12...
A similar topic here mentions it as well : http://www.sciencemadness.org/talk/viewthread.php?tid=31537
There is also a method on page 596 of Vogel's 3rd edition that discusses the three methods to accomplish this reduction of diazonium salts,
hypophosphorus acid, ethanol, and alkaline sodium stannite(I apologize for a typo in my previous post, it is stannite and not
stannate). It appears that ethanol is not favorable due to potential formation of ethyl ethers, thus sodium stannite or hypophosphorus acid are
preferred.
[Edited on 5-12-2015 by gdflp]
|
|
|
Molecular Manipulations
Hazard to Others
  
Posts: 447
Registered: 17-12-2014
Location: The Garden of Eden
Member Is Offline
Mood: High on forbidden fruit
|
|
Hmm, still neither mentioned anything besides arenediazonium, in less detail and with no references than Wiki . .
Anything regarding straight chain compounds? I'm very interested in this.
-The manipulator
We are all here on earth to help others; what on earth the others are here for I don't know. -W. H. Auden
|
|
|
Leo Szilard
Harmless

Posts: 20
Registered: 10-5-2015
Location: USA
Member Is Offline
Mood: No Mood
|
|
Thanks for all of the replies. Hearing that these tend to be "dirty reactions" is an example of some of the insight that is interesting to me. In
textbooks, you rarely get good insight into practical considerations such as ease of workup. Articles, too, often gloss over a lot of details (for the
sake of consision, I suppose).
So clearly diazoniums are unstable--by "design" I suppose. It seems to me that they should be almost explosively unstable--N2 is a fantastic leaving
group. I would expect them to be difficult and/or dangerous to work with. Is that true?
|
|
|
Molecular Manipulations
Hazard to Others
  
Posts: 447
Registered: 17-12-2014
Location: The Garden of Eden
Member Is Offline
Mood: High on forbidden fruit
|
|
Not explosive. What would the carbon bond to if nitrogen left? Diazonium, being positively charged, it needs to reduce the carbon, which means
something must replace it.
Unstable? Yes. The first time I did the same reaction (posted above) I gave the RBF a small swirl after all the hydrochloric acid was added. This
caused more bubbles to form, in turn creating more nucleation sites for more bubbles to form. The decomposing reaction, being rather exothermic began
to 'run away', spewing hot nitrogen and nitrogen oxides. It went from 0 degrees. C to boiling in less than thirty seconds.
[Edited on 12-5-2015 by Molecular Manipulations]
-The manipulator
We are all here on earth to help others; what on earth the others are here for I don't know. -W. H. Auden
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Quote: Originally posted by Molecular Manipulations  | Not explosive. What would the carbon bond to if nitrogen left? Diazonium, being positively charged, it needs to reduce the carbon, which means
something must replace it.
|
Yes, they certainly are explosive if the solvent is left to evaporate. The nitrogen leaves as N2 and the anion bonds to the carbon. Alkyl
diazonium salts are typically too reactive to be useful, as they tend to rearrange and are extremely unstable(the nitrogen typically leaves before a
reaction can be performed), so I have doubts as to whether this reduction can be done on these substrates.
|
|
|
Molecular Manipulations
Hazard to Others
  
Posts: 447
Registered: 17-12-2014
Location: The Garden of Eden
Member Is Offline
Mood: High on forbidden fruit
|
|
They disproportionate IIRC and form azides, which are highly explosive. However over 5 degrees C they just decompose into nitrogen.
Your right though. I forgot about the damn anion! Diazonium ions, like any cation only exist with an anion to balance the charge...
Most react with water, although it seems to take longer than I first thought (weeks ago).
[Edited on 12-5-2015 by Molecular Manipulations]
-The manipulator
We are all here on earth to help others; what on earth the others are here for I don't know. -W. H. Auden
|
|
|
Leo Szilard
Harmless

Posts: 20
Registered: 10-5-2015
Location: USA
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by gdflp  | Quote: Originally posted by Molecular Manipulations  | Not explosive. What would the carbon bond to if nitrogen left? Diazonium, being positively charged, it needs to reduce the carbon, which means
something must replace it.
|
Yes, they certainly are explosive if the solvent is left to evaporate. The nitrogen leaves as N2 and the anion bonds to the carbon. Alkyl
diazonium salts are typically too reactive to be useful, as they tend to rearrange and are extremely unstable(the nitrogen typically leaves before a
reaction can be performed), so I have doubts as to whether this reduction can be done on these substrates. |
Granted I haven't seen loads of examples, but I have only ever read about aryl substrates.
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Ummm. Generally OK in solutions. Fairly stable as Fluoroborate Salts, but a good rule of thumb is to avoid working with Diazobenzenes as solids.
Blow you off the face of the planet, if something goes wrong.
Diazomethane is a very useful reagent, which none-the-less is very seldom used. Little things like the presence of a scratch on the inner surface
your glassware (or the presence of a ground glass joint), can cause detonation.
Been some useful reagents produced via the diazotization of Aminoacids.
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Not to mention that diazomethane is quite toxic.
|
|
|
Leo Szilard
Harmless

Posts: 20
Registered: 10-5-2015
Location: USA
Member Is Offline
Mood: No Mood
|
|
I think it would be fun to prepare some diazo dye compounds. Additionally, Sandmeyer reactions seem synthetically very useful so I want to investigate
it more.
In school, I was taught that if (aryl) diazonium salts are heated, you form an aryl carbonation to which you can add a nucleophile. The problem that I
have with this explanation is that it doesn't explain why copper (i) compounds are used. E.g. introduction of a chloride calls for CuCl. If it were
really as simple as an "sn1"-like reaction, any chloride source should be fine. I suspect that there is some SET process going on. Any thoughts?
|
|
|