| Pages:
1
2 |
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Very First Step into Organic Chemistry
Can anyone suggest a practical experiment (preferably with a reference) that would offer a Way Into OC ?
Personally i find if baffling, and cannot see a way in.
Yes, i've done some OC, and understand not one iota of it.
Today some linseed oil decided to puff smoke at me, and i really really would like to have some handle on the Why.
To be fair, the oil had been treated badly with SCl2 and a bit of ammonia, so it had just cause.
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
One of the first organic experiment I attempted at home was a Fisher esterification of ethanol and acetic acid to produce ethyl acetate.
Its a common example for the Fischer esterification.
Cheap and easy to handle reagents.
[Edited on 28-3-2015 by Loptr]
|
|
|
Etaoin Shrdlu
National Hazard
   
Posts: 724
Registered: 25-12-2013
Location: Wisconsin
Member Is Offline
Mood: Insufferable
|
|
Dehydration of a secondary or tertiary alcohol to make alkenes, that's pretty simple, and easy to see you've succeeded because the products distill at
a much lower temperature than the alcohol.
http://www.nvcc.edu/alexandria/stb/chm/245/45_dehydration.pd...
[Edited on 3-28-2015 by Etaoin Shrdlu]
|
|
|
Molecular Manipulations
Hazard to Others
  
Posts: 447
Registered: 17-12-2014
Location: The Garden of Eden
Member Is Offline
Mood: High on forbidden fruit
|
|
The first and most important step is to learn organic nomenclature, without this every IUPAC name, every chemical structure and every functional group
will be a complete mystery. It's super easy and logical.
As for experiments, making an ester is fun. All you need is an alcohol and sulfuric acid. Nitrations are fun and yield a product that has energetic
properties.
Just curious, how many of you recognize this off the bat?
-The manipulator
We are all here on earth to help others; what on earth the others are here for I don't know. -W. H. Auden
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Quote: Originally posted by Molecular Manipulations  | The first and most important step is to learn organic nomenclature, without this every IUPAC name, every chemical structure and every functional group
will be a complete mystery. It's super easy and logical.
As for experiments, making an ester is fun. All you need is an alcohol and sulfuric acid. Nitrations are fun and yield a product that has energetic
properties.
Just curious, how many of you recognize this off the bat? |
Hell no, but please explain. I am still a novice with nomenclature.
|
|
|
Etaoin Shrdlu
National Hazard
   
Posts: 724
Registered: 25-12-2013
Location: Wisconsin
Member Is Offline
Mood: Insufferable
|
|
Not going to show stereoisomerism, MM? 
[Edited on 3-28-2015 by Etaoin Shrdlu]
|
|
|
Molecular Manipulations
Hazard to Others
  
Posts: 447
Registered: 17-12-2014
Location: The Garden of Eden
Member Is Offline
Mood: High on forbidden fruit
|
|
What for that molecule? Stereoisomers are isomeric molecules that have the same molecular formula and sequence of bonded atoms (constitution), but
that differ only in the three-dimensional orientations of their atoms in space. - Wikipedia.
It would take up way too much space just to quote a guide to nomenclature, it's not complicated, but there's a lot of definitions to memorize. Here's
a link to a guide http://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/nome...
That's a rather well known molecule, at least around here
[Edited on 28-3-2015 by Molecular Manipulations]
-The manipulator
We are all here on earth to help others; what on earth the others are here for I don't know. -W. H. Auden
|
|
|
Lambda-Eyde
National Hazard
   
Posts: 860
Registered: 20-11-2008
Location: Norway
Member Is Offline
Mood: Cleaved
|
|
You don't think there are any stoners on the forum? 
This just in: 95,5 % of the world population lives outside the USA
Please drop by our IRC channel: #sciencemadness @ irc.efnet.org
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
aga:
Look at that chemistry book you bought. It has examples of most basic OC reactions and mechanisms. In many cases, like the on ES linked to, you'll
find classroom experiments completely laid out for you.
I'd certainly recommend Fisher esterifications. Lovely smells if you make the right ones, easy to do and need basic work up techniques that are useful
later on.
|
|
|
Etaoin Shrdlu
National Hazard
   
Posts: 724
Registered: 25-12-2013
Location: Wisconsin
Member Is Offline
Mood: Insufferable
|
|
The "one everyone thinks of" is a specific stereoisomer. It was a joke. xP
[Edited on 3-28-2015 by Etaoin Shrdlu]
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Oh, I get it now.
Sorry, I dont, as it goes with the job... Not to mention I have too many distractions in life, and dont need another.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
DOH !
You mean i have to Work to understand it ?
This internet thing isn't living up to expectations.
|
|
|
morganbw
National Hazard
   
Posts: 561
Registered: 23-11-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Molecular Manipulations  | The first and most important step is to learn organic nomenclature, without this every IUPAC name, every chemical structure and every functional group
will be a complete mystery. It's super easy and logical.
|
I have some/much to learn. Your advise is dead on. It lays the foundation that is required to understand anything pertaining to organic chem.
Thank you sir, you reminded me of what I knew to be but have been lazy/sidetracked away from.
|
|
|
morganbw
National Hazard
   
Posts: 561
Registered: 23-11-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | DOH !
You mean i have to Work to understand it ?
This internet thing isn't living up to expectations. |
I am not there yet, but I am thinking that it is not really that much work.
Just a different challenge which if not taken will keep me behind where I want to be.
|
|
|
j_sum1
Administrator
       
Posts: 6326
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
I think I am in the same position as you aga. I know nearly no OC.
I have done esterifications, oxidation of alcohols and halogenation of unsaturated hydrocarbons.
I know about simple nomenclature, some of the more basic functional groups, isomerism, polymers and some general principles for correlating
structural formula with physical properties and (some) chemical properties.
Get me into aromatics or start talking about alpha carbons or ask me to choose a catalyst or break a C-C bond and the wheels fall off.
I am going to make oil of wintergreen from aspirin with my class in a few weeks -- and that is mostly to get some experience with some new techniques.
I am going to follow MrHomeScientist's youtube clip. It looks straightforward and fun.
And since someone else posted it recently, here is an elementary road map for the field. It might be useful.
http://www.compoundchem.com/infographics/#orgchem
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
Have you looked for organic chemistry lab books? Some are available online for various college courses, and have experiments one can perform at home
with mechanisms and theory, designed to be learned alongside textbook readings.
Esterification can be fun and rewarding.
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
Like others have said, learning nomenclature, stereochemistry, and how to interpret skeletal structures and functional groups is a must. Being able to
follow the flow of electrons (electron pushing) in a mechanism is necessary, too. Honestly, in addition to those things, I'd also start by learning
about nucleophiles and electrophiles, specifically what they are and how to differentiate between them. Being able to correctly identify the
nucleophile(s) and electrophile(s) in a reaction may very well be the single most important step in actually understanding it.
Start with some basic substitution and elimination reactions first. Try to stick to the more simple reactions of each type until it starts making
sense conceptually, then slowly progress to more and more complex ones. For example, with the nucleophilic substitutions, start with very simple
SN1 and SN2 reactions first. Once you start getting the hang of it, you can then move on to slightly more complex kinds of
substitution, such as intramolecular substitution (ring closure) and those that involve an intimate ion pair (SNi).
For the time being, focus mostly on simple reactions whose mechanisms are known and have been studied for a long time. Forget about trying to make
sense of reactions with mechanisms that aren't very well understood, for instance a lot of dissolving metal reductions and various other one-electron
reductions involving free radicals and single-electron transfer.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
There a four classical reaction classes in organic chemistry, [1]addition, [2]substitution, [3]ellimination and [4]radical reactions, each have a few
further subsets. After learning the basics about how to name and draw organic compounds, you can work your way through the theory of each of those
reaction types and then try your hand at performing a reaction that is representative of each. That would be an excellent start.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Thanks for the pointers.
Making ethyl acetate looks do-able, and has some indicator of success (smell) so i'll give that a go.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | Thanks for the pointers.
Making ethyl acetate looks do-able, and has some indicator of success (smell) so i'll give that a go. |
Methyl salicylate, very simple version:
https://www.youtube.com/watch?v=Nu2Excsv4zE
Salicylic acid = aspirin, so very OTC.
Also easy and lovely smelling: methyl benzoate. And if you have sodium benzoate then getting benzoic acid from it is a doddle.
[Edited on 29-3-2015 by blogfast25]
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
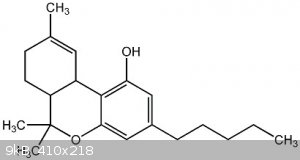
that long n-pentyl side chain on the aromatic ring makes it look like a cannabinol derivative
[Edited on 29-3-2015 by CuReUS]
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by CuReUS  |
that long n-pentyl side chain on the aromatic ring makes it look like a cannabinol derivative
[Edited on 29-3-2015 by CuReUS] |
It is indeed (+/-)-delta-9-tetrahydrocannabinol. I made a few hundred grams of an ("inactive") analogue under Home Office license during previous
employment. I wouldn't recommend it as a first attempt at organic synthesis though.
[Edited on 29-3-2015 by DJF90]
|
|
|
brubei
Hazard to Others
  
Posts: 188
Registered: 8-3-2015
Location: France
Member Is Offline
Mood: No Mood
|
|
Performing organic synthesis is pleasant when you can identify your product apparition and deseappearition. Learning TLC monitoring is a good way to
do that.
then TLC can be used for simple analytic technique with almost every thing you can find. Searching for alcaloid, protein, oils, etc in food, flower
...
here is a good textbook, you can learn to recognize the organic function in a molecule, maybe how they react.
http://www.clubdeccm.com/PDF/Dyeing_Reagents_TLC.pdf
[Edited on 29-3-2015 by brubei]
|
|
|
BromicAcid
International Hazard
    
Posts: 3248
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
I did inorganic synthesis for years in my back yard and it was strange when I had to actually do an organic reaction. At the time I thought I was
going to grow up and become an inorganic chemist (college taught me that was a lot different than I would have hoped) so I really didn't want to even
try orgo. I remember my first synthesis clearly, nitrobenzene. Easy to conduct, easy to purify, excellent (headache-inducing) smell. The motivation
was purely to get a solvent to carry out the electrolysis of NaCl/AlCl3 mixture. Out of hundreds of home chemistry experiments I probably only
performed a dozen or so organic transformations. Sure, a nitration can seem like a reaction for all the energetics people out there but the standard
organic chemist does have reason to fish the reaction out of their repertoire from time to time. Yields are good, I would certainly recommend it to
others as a first experiment, plus if you don't have benzene you get to make that first, another exciting complication.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Just finished having a go at Ethyl Acetate.
Had to make Acetic Anhydride first, so it took a while.
( 30ml Ethanol close to anhydrous, 30ml Acetic Anhydride, 6ml 98% H2SO4, reflux 30 mins)
Must have worked as the smell is now that of NVR instead of vinegar, however it won't separate from the 'neutralised' reaction mixture.
Some questions :-
If you're going to reflux with heating, is there any point cooling the mixture as you add the acid ?
Is it necessary to neutralise the acid after the reaction ?
|
|
|
| Pages:
1
2 |