nelsonB
Hazard to Self
 
Posts: 71
Registered: 5-9-2013
Member Is Offline
Mood: No Mood
|
|
Nitrocyclohexane Synthesis Via double displacement reaction
Hi
so i have kind of new to organic chemistry
i am using ChemSketch
considering mixing 1 moles of Sodium nitrite with 1 moles of sodium cyclohexyl Sulfate, could we obtain Nitrocyclohexane in any way

[Edited on 28-3-2015 by nelsonB]
[Edited on 28-3-2015 by nelsonB]
[Edited on 28-3-2015 by nelsonB]
|
|
|
mnick12
Hazard to Others
  
Posts: 404
Registered: 30-12-2009
Location: In the lab w/ Dr. Evil
Member Is Offline
Mood: devious
|
|
Thats not going to work.
It looks like you are proposing an SN2 reaction.
A couple of things to consider:
How well does the cyclohexyl moiety participate in SN2? Hint: very poorly
How good of a nucleophile is nitrite? Again pretty darn bad
Also where is the negative charge on nitirite? If it did work you would not get the N-C bond you are looking for.
How good of a leaving group is the sulphonate?
Also posts without a reference or some experimental data should be posted in the beginings section.
I honestly don't know how it is made commercially, but I dont think its a substitution reaction. Try looking in the lit, im sure there is a prep
somewhere.
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
As said, no, and definitely this is Beginnings level at best. I suggest looking more into a textbook to clear up some obvious misconceptions like
functional groups and SN2 reactivity. If you are looking to nitrate something, it would probably be best to look at the mechanisms that describe the
reaction before proposing something new without an accompanying mechanism. If you would like, we could all make book suggestions as there is a pretty
good stickied thread.
It is nice to see you trying to teach yourself, and even using software for drawing, though!
|
|
|
Bert
|
Thread Moved
28-3-2015 at 04:25 |
nelsonB
Hazard to Self
 
Posts: 71
Registered: 5-9-2013
Member Is Offline
Mood: No Mood
|
|
as i see anyway writing a bunch of molecule for an example is a bit stupid here i cleared the exemple for a simpler one and i reduced the size of the
picture in case this was about why its have been removed from the image tag
Is the exemple better ?
|
|
|
Etaoin Shrdlu
National Hazard
   
Posts: 724
Registered: 25-12-2013
Location: Wisconsin
Member Is Offline
Mood: Insufferable
|
|
Hmmmm, I'm not as sure as you are that this is bunk. I thought one could make nitroalkanes by reaction of nitrite salts
with haloalkanes. Wikipedia is indicating it works with alkyl sulfates as well but the source is in German and I can't tell how well (or if) it
supports the conclusion.
Walden, P. (1907). "Zur Darstellung aliphatischer Sulfocyanide, Cyanide und Nitrokörper". Berichte der deutschen chemischen Gesellschaft 40 (3):
3214–3217. doi:10.1002/cber.19070400383
Ignore the above, I forgot sulfate sticks hard to carbon on the C-S bond and it's the sulfonate esters that will leave nicely.
I know for sure nitroalkanes can be synthesized using silver nitrite and alkyl iodides/bromides, which isn't too terribly far off from what what's
being proposed. I don't know what kind of yields are possible; it's one of the steps in the Victor Meyer test to distinguish
primary/secondary/tertiary alcohols and I'm not very familiar with it. Also, I think alkyl nitrites are produced as well depending on substrate.
Probably not impossible with a better leaving group.
[Edited on 3-28-2015 by Etaoin Shrdlu]
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
This is a little better, especially since you no longer have sulfates attached to amines (nitrogen at the oxygen) and sp carbons with three oxygens
attached.
Normally you show catalysts over the reaction arrows, so we have to assume there are none being used.
|
|
|
mnick12
Hazard to Others
  
Posts: 404
Registered: 30-12-2009
Location: In the lab w/ Dr. Evil
Member Is Offline
Mood: devious
|
|
Quote: Originally posted by Etaoin Shrdlu  | Hmmmm, I'm not as sure as you are that this is bunk. I thought one could make nitroalkanes by reaction of nitrite salts
with haloalkanes. Wikipedia is indicating it works with alkyl sulfates as well but the source is in German and I can't tell how well (or if) it
supports the conclusion.
Walden, P. (1907). "Zur Darstellung aliphatischer Sulfocyanide, Cyanide und Nitrokörper". Berichte der deutschen chemischen Gesellschaft 40 (3):
3214–3217. doi:10.1002/cber.19070400383
Ignore the above, I forgot sulfate sticks hard to carbon on the C-S bond and it's the sulfonate esters that will leave nicely.
I know for sure nitroalkanes can be synthesized using silver nitrite and alkyl iodides/bromides, which isn't too terribly far off from what what's
being proposed. I don't know what kind of yields are possible; it's one of the steps in the Victor Meyer test to distinguish
primary/secondary/tertiary alcohols and I'm not very familiar with it. Also, I think alkyl nitrites are produced as well depending on substrate.
Probably not impossible with a better leaving group.
[Edited on 3-28-2015 by Etaoin Shrdlu] |
I know there is that rhodium page everyone seems to cite on synthesizing nitroethane from ethyl bromide and either sodium or silver nitrite.
It might work with the silver salt, but I doubt it would with sodium nitirite. Additonaly, as you mentioned, the alkyl nitrite would be a major
product. In the end I dont think one could get good yields from this reaction, though you may be able to get something if you use silver nitrite.
|
|
|
Etaoin Shrdlu
National Hazard
   
Posts: 724
Registered: 25-12-2013
Location: Wisconsin
Member Is Offline
Mood: Insufferable
|
|
Apparently, sodium nitrite in DMF works great for bromocyclopentane, and great for bromocycloheptane, annnnnnd
bromocyclohexane just gives cyclohexane as a product. Table 2.3, it's a page further.
On a similarly surprising note, silver nitrite seems to work the best for primary alkyl halides, but for secondary and tertiary it's terrible and
sodium nitrite works fine.
Chemistry Gods, what have you been smoking?
EDIT: Looks like a couple of the listed reactions might be catalyzed by sodium iodide. Too bad I really don't want the desired product around, or I'd
try it.
[Edited on 3-28-2015 by Etaoin Shrdlu]
|
|
|
mnick12
Hazard to Others
  
Posts: 404
Registered: 30-12-2009
Location: In the lab w/ Dr. Evil
Member Is Offline
Mood: devious
|
|
Quote: Originally posted by Etaoin Shrdlu  | Apparently, sodium nitrite in DMF works great for bromocyclopentane, and great for bromocycloheptane, annnnnnd
bromocyclohexane just gives cyclohexane as a product. Table 2.3, it's a page further.
On a similarly surprising note, silver nitrite seems to work the best for primary alkyl halides, but for secondary and tertiary it's terrible and
sodium nitrite works fine.
Chemistry Gods, what have you been smoking?
EDIT: Looks like a couple of the listed reactions might be catalyzed by sodium iodide. Too bad I really don't want the desired product around, or I'd
try it.
[Edited on 3-28-2015 by Etaoin Shrdlu] |
Thanks for the link,
It looks like the yields reported for the cyclic bromoalkanes and NaNO2 are 0-61%, which I would not call "great" but acceptable.
More importantly for the OP, the reported yield for bromocyclohexane was 0%.
I would be interested to hear if anyone has done reactions like this, and can confirm the reported yields.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by mnick12  |
How good of a nucleophile is nitrite? Again pretty darn bad
Also where is the negative charge on nitirite? If it did work you would not get the N-C bond you are looking for. |
The nitrite ion is actually a relatively good nucleophile in aprotic media. Like most poorly polarizable nucleophiles it is a poor nucleophile in protic media, but becomes
much more reactive in aprotic solvents.
The formal position of the negative charge is not the only factor that dictates the O- vs. N-alkylation selectivity. The localization of the charge is
just a formality, an oversimplified model, that does not always properly represents real systems. In the case of the nitrite anion, both the electron
pairs on oxygen and the one on the nitrogen can participate in the SN2 substitutions. In principle, softer electrophiles tend to give more
N-alkylation, but a lot also depends on the counterion and the solvent.
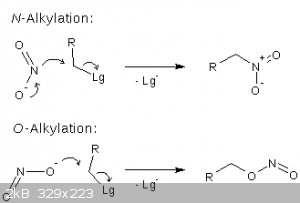
Synthesis of nitromethane and nitroethane from methyl and ethyl sulfates and NaNO2 is discussed in many threads. At least one thread describes a
detailed experimental.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
If you have cylcohexane and diluted HNO3...you may get nitrocyclohexane in good yield, aside with cyclohexanone oxydation byproduct by thermic
nitration in vapor phase at a relatively moderate temperature (120 < x < 350°C) but I don't remember the temperature.
[Edited on 31-3-2015 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
nelsonB
Hazard to Self
 
Posts: 71
Registered: 5-9-2013
Member Is Offline
Mood: No Mood
|
|
PHILOU Zrealone
should not nitrating with regular nitric acid yield something like
1,3,5-Trinitrocyclohexane
or giving a random nitro group on the molecule ?
|
|
|