| Pages:
1
2 |
YT2095
International Hazard
    
Posts: 1091
Registered: 31-5-2003
Location: Just left of Europe and down a bit.
Member Is Offline
Mood: within Nominal Parameters
|
|
hydroquinone/picric acid
as the quinone is basicly Phenol with an extra OH at 5, it seemed reasonable to me that picric acid shouldn`t be all that dificult to make from it
(less work than some of the crude ASA synths).
I worked from the starting point of having my pure ASA and been heated with conc H2SO4 liberating the acetic acid etc...
and just added conc H2SO4 to 50mg of Quinone.
I let that stand for most of the afternoon as I had work to do, when I came back there was a tiny tiny darkening of the soln and the Quinone was all
foating on the top.
I added a few crystals of KNO3 (about 200 mg) to the approx 3ml of soln, no reaction at 1`st and then slowly the NO2 evolved, the liquid layer
started to go yellow and the needle like crystals took on a black(ish) color.
there was no noticable heat created (to the hand) and certainly no runaway nitration.
after 15 mins of standing with a stir every few mins, the liquid layer is getting more Orange and there`s less Black crystals.
my questions are...
1) why did they go black?
2) is this in anyway a Viable method of Picric acid formation as I thought it would be?
3) if it Does form Picric acid, what`s the best way to dispose of it (and Don`t say blow it up! that exactly what I Don`t want to do).
\"In a world full of wonders mankind has managed to invent boredom\" - Death
Twinkies don\'t have a shelf life. They have a half-life! -Caine (a friend of mine)
|
|
|
DeAdFX
Hazard to Others
  
Posts: 339
Registered: 1-7-2005
Location: Brothel
Member Is Offline
Mood: @%&$ing hardcore baby
|
|
Styphnic acid has two hydroxyl's if I remember correctly but is made from rescorniol(sp) and not hydroquinone. Maybe you have a different isomer of
styphnic acid?
|
|
|
YT2095
International Hazard
    
Posts: 1091
Registered: 31-5-2003
Location: Just left of Europe and down a bit.
Member Is Offline
Mood: within Nominal Parameters
|
|
if mem serves me corectly I think the propper name is something like 1,4 para benzene diol.
basicly it`s just Phenol with another OH group on the opposing side of the ring.
catechol`s also similar but that`s like 1,2 benzene diol, that has the 2 OHs side by side.
(I`m working from mem, and although corrections are welcome, the above has little relevance, the 2 OH`s Do though).
I`m not sure Quinone can Have an Isomer in the way of Cis Trans anyway?
non the less, the soln is now a deep orange, and strongly ressembles picric acid in color. I`m still curious at to the questions I posted.
\"In a world full of wonders mankind has managed to invent boredom\" - Death
Twinkies don\'t have a shelf life. They have a half-life! -Caine (a friend of mine)
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Most nitroaromatics are yellowish in colour. I don't believe you have formed picric as that would mean loss of an OH group, which is not so easy.
You likely have a mixture of nitrated hydroquinone products mainly di and mono, with perhaps a small ammount of tri.
[Edited on 8-7-2006 by rogue chemist]
|
|
|
YT2095
International Hazard
    
Posts: 1091
Registered: 31-5-2003
Location: Just left of Europe and down a bit.
Member Is Offline
Mood: within Nominal Parameters
|
|
Thanks, and yes, that makes sense, sadly I think I`ve left it a little too long now as the contents are entirely Black.
the Quinone with the Conc H2SO4 and KMnO4 also went black over night, the only reasonable reaction was the Quinone in water with Iodine crystals.
I`m sure I`ll find something interesting to do with this chem eventualy, other than make it go Black!
\"In a world full of wonders mankind has managed to invent boredom\" - Death
Twinkies don\'t have a shelf life. They have a half-life! -Caine (a friend of mine)
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
Hydroquinone is very easily oxidised and tends to fall apart. If you think how much more reactive and easily oxidised phenol is than benzene, add an
extra OH, then factor in the stability of the conjugation of the quinone oxidation product...
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by YT2095
as the quinone is basicly Phenol with an extra OH at 5, it seemed reasonable to me that picric acid shouldn`t be all that dificult to make from it
(less work than some of the crude ASA synths). |
Quinone is not a phenol and has no OH groups. I assume you mean p-benzoquinone as that is the only quinone shortened to simply "quinone". Or
perhaps you have confused quinone with hydroquinone which is indeed a phenol, but "4-hydroxyphenol" and not 5-.
Anyway, there is no reasonable synthetic route to turn either quinone or hydroquinone to picric acid.
| Quote: | | 1) why did they go black? |
I don't understand what you mean. Quinone is already black (well it can be anything from dark brown to dark green). In case you used the colorless
hydroquinone (since you talk about "needle like crystals" and don't mention them having a color) then it is obvious they went black as all kind of
shit happened to them. Hydroquinone easily oxidizes to quinone and other tarry crap (all black), especialy in the presence of HNO3 (to crap mostly).
| Quote: | | 2) is this in anyway a Viable method of Picric acid formation as I thought it would be? |
No, check the structure of p-benzoquinone, hydroquinone and picric acid. If you would have enough knowledge of organic reactions you would realize that immediately by yourself.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Of course he meant hydroquinone.
What is interesting that resorcinol is 3-hydroxy phenol, so very similar to hq. From this stypnic acid can be made like a charm.
I was thinking, perhaps the best way to make energetic derivatives form this is to first methylate the hydroxyquininone, to i.e. 1,4 methoxy benzene
using any of the standard methylating agents. This then could be nitrated. The question is, since both -OCH3's are activating, would one get the
tetranitro derivative of 1,4 methoxy benzene, or always and exclusively, either the 2,5 or the 36 di-nitro derivatives? or only 2, 6 and 3, 5 dinitro
derivatives?
So yes there are things that can be done with hydroquinone.
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
YT2095
International Hazard
    
Posts: 1091
Registered: 31-5-2003
Location: Just left of Europe and down a bit.
Member Is Offline
Mood: within Nominal Parameters
|
|
Fascinating! I have 1,2 benzene diol also (or is it worded; 2, hydroxyphenol?) that sounds very similar too.
I`m trying to teach myself a little Organic Chem with the aid of a few good text books and some basic compounds, so I`m a relative Newb in this area.
I wanted Picric acid as it`s used as a dye (or at least was once upon a time), ultimately I`de like to work towards making Azo Dyes and some
interesting esters.
did anyone know that trinitro methylbenzene is actualy used in some perfumes!
anyway, I`ll try the same experiments with the pyrocatechol and see what happens as well as look up this Stypnic acid.
\"In a world full of wonders mankind has managed to invent boredom\" - Death
Twinkies don\'t have a shelf life. They have a half-life! -Caine (a friend of mine)
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
| Quote: | Originally posted by YT2095
did anyone know that trinitro methylbenzene is actualy used in some perfumes!
|
You sure about that? That is plain ol TNT, and with its toxicity and staining ability(similar to nitrophenols but not as strong IIRC) I am surprised
it is in anything that would get put on the skin.
[Edited on 10-7-2006 by rogue chemist]
|
|
|
YT2095
International Hazard
    
Posts: 1091
Registered: 31-5-2003
Location: Just left of Europe and down a bit.
Member Is Offline
Mood: within Nominal Parameters
|
|
Yup 100% certain, it`s used as a Musk compound.
"The discovery of the first synthetic musks is a by product of research on explosives. In 1888, Albert Baur, in the process of searching for new
explosives noticed that the product of reaction of trinitrotoluene (TNT) and tert-butyl halides produced a pleasant odour. Musk Baur became the first
synthetic musk, classified under the nitro musks category. In 1894 he produced Musk Ketone, which was said that resemble the natural musk fairly
closely and until quite recently was among the popular perfume ingredients. The nitro musks (Musk Xylol, Musk Ketone, Musk Tibetene, Musk Ambrette,
Moskene) possess a warm, powdery scent, with an ambery and animalic overlay. The sensual warmth of Musk Ketone as well as other nitro musks pervades
the base of Chanel No. 5 (1921) and of many other fragrances from that period. "
Taken from: http://boisdejasmin.typepad.com/_/2005/11/fragrance_ingre.ht...
there`s probably 100`s of other sources for a TNT Musk search too.
here`s another: http://www.matze-wormuth.de/musk.htm
[Edited on 10-7-2006 by YT2095]
\"In a world full of wonders mankind has managed to invent boredom\" - Death
Twinkies don\'t have a shelf life. They have a half-life! -Caine (a friend of mine)
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | | The question is, since both -OCH3's are activating, would one get the tetranitro derivative of 1,4 methoxy benzene, or always and exclusively, either
the 2,5 or the 36 di-nitro derivatives? or only 2, 6 and 3, 5 dinitro derivatives? |
The only reference for 2,3,5,6-tetranitro-1,4-dimethoxybenzene is J. Chem. Soc. C, 1967, 1235 - 1238. They nitrated 2,3,5-trinitro-4-methoxyphenol
or 3,5-dinitro-4-methoxyphenol in HNO3 (d = 1.4 g/ml) at 70°C. Then they methylated the resulting 2,3,5,6-tetranitro-4-methoxyphenol with dimethyl
sulphate.
The paper is attached.
1,4-dimethoxy benzene can be mononitrated and dinitrated (forming a mixture of 2,5 and 2,3 dinitro regioisomers). I have not seen any reference for
trinitration but I have never searched anyway. Perhaps it might be even possible to tetranitrate it directly without extensive demethylation, but it
seams like it has not been done yet.
| Quote: | did anyone know that trinitro methylbenzene is actualy used in some perfumes!
Yup 100% certain, it`s used as a Musk compound. |
If you would read the text you cited a bit more carefuly you would notice that there is no mention of TNT being used as a perfume. It only says that
TNT was used as a reagent to prepare musk like smelling compounds.
[Edited on 10-7-2006 by Nicodem]
Attachment: J39670001235.pdf (598kB)
This file has been downloaded 982 times
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
YT2095
International Hazard
    
Posts: 1091
Registered: 31-5-2003
Location: Just left of Europe and down a bit.
Member Is Offline
Mood: within Nominal Parameters
|
|
I read and cited them Very carefully thank you, what part didn`t you understand?
it is (or at least was) used in perfume making. and no, no single perfume Ever is just a single compound, they use a base(s) to start with and then
select the over-tones.
I fail to see your point here? Unless it`s one based upon nit-picking or semantics? I`m good at neither of those.
\"In a world full of wonders mankind has managed to invent boredom\" - Death
Twinkies don\'t have a shelf life. They have a half-life! -Caine (a friend of mine)
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
Govermental disposal of nitrated phenolsls is somewhat complex. Regulations differ but in general the material needs to be broken down as then
disposed as a toxic waste. It is broken down at a waste facility designed for that purpose.
If the intention is to dispose of a small amount this could be accomlished by the addition of a chlorinated salt to a high concentration solution. But
in a situation wherein there is concern for environmental issues and one is disposing of a large amount then any toxic waste facility would be
appropriate for containerized nitrophenols. An addition of chlorobenzene to the completed tri-nitrated form will yield a non-explosive material that
may be diluted and disposed of. I think you can find that in the PATR.
As a dye it has been found (and many who have experimented w/ PA will attest) that the higher the pH, the redder the material. An orange PA indicated
that there exists a higher pH in the production. this can be taken to extremes and a "red-goo" material achieved. The issue here is that what some had
thought to be a method of making PA non-explosive by raising the pH very high didn't work for the most part. I am not sure that all benzene-ring
explosives behave like TNT but in that case the addition of an after productiion hydroxide will sensitize it!
|
|
|
YT2095
International Hazard
    
Posts: 1091
Registered: 31-5-2003
Location: Just left of Europe and down a bit.
Member Is Offline
Mood: within Nominal Parameters
|
|
Turmeric, another Dye found in the root of a certain plant will also dye clothes a remarkably similar color to PA, interestingly enough, it Also will
go red the greater the PH. this can also happen Long after the item of clothing ahs been worn and washed a kazillion times, drop some NaOH on it for
instance and Wallop, bright red 
Sunlight tends to fade it though, so the next curry stain you get on your best T`shirt, shove it under a sunlamp for a while 
\"In a world full of wonders mankind has managed to invent boredom\" - Death
Twinkies don\'t have a shelf life. They have a half-life! -Caine (a friend of mine)
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
| Quote: | | The discovery of the first synthetic musks is a by product of research on explosives. In 1888, Albert Baur, in the process of searching for new
explosives noticed that <b>the product of reaction of trinitrotoluene (TNT) and tert-butyl halides</b> produced a pleasant odour. Musk
Baur became the first synthetic musk, classified under the nitro musks category. In 1894 he produced Musk Ketone, which was said that resemble the
natural musk fairly closely and until quite recently was among the popular perfume ingredients. The nitro musks (Musk Xylol, Musk Ketone, Musk
Tibetene, Musk Ambrette, Moskene) possess a warm, powdery scent, with an ambery and animalic overlay. The sensual warmth of Musk Ketone as well as
other nitro musks pervades the base of Chanel No. 5 (1921) and of many other fragrances from that period. |
It sounds like only a derivative of TNT is used, not the explosive itself.
|
|
|
Mason_Grand_ANNdrews
Hazard to Self
 
Posts: 63
Registered: 19-1-2006
Location: New Berlin City !!
Member Is Offline
Mood: crabby
|
|
I wonder, this method will be most interestingly, "qiunone" to phenol. I`ve some way obtaining dipicric acid. This wil formed by the way of
benzophenone but i`m not shure.
This will describe the method:
Prepare a solution Benzophenone in Methanol and to this a solution of NaOH will be added. This is dilluted with a large amount of 3% HCl to
precipitate the benzophenol. I don`t know the result is slight soluble in water and this are right, at least a precipitation will be possible when the
mix is cooled to 0 °C. I will calcultale the amounts later, possibly ethanol is used for this. The nitrated benzophenol should give
2,2`,4,4`,6,6`-hexanitrobenzophenol or dipicric acid.
1. the result is not slight solule in water
Prepare a mix of 500 ml 98% H2SO4 and 340 ml 70% HNO3 in a cold water bad. To this 20 g of benzophenol is careful stirred in and he mixture is than
over a period of 4 hours stirred and slowly heated to 100 °C. to this 2000 ml cold water is added, the product is filtered and a washed with a large
amount of water wich will remove the H2SO4 of the product. This will be similar to the process of 4-chloro-2,6-dinitrophenol and will use a little
waste of H2SO4.
2. the result is slight soluble in water
240 ml of 98% H2SO4 and 340 ml 70 % HNO3 will be enough,
but the benzophenol is nitrated complete ? 1000 ml water is added to the cooled mix and the filtered result is washed with small portions of water.
To obtain a better result fuming concentrated H2SO4 is used. A nitrated result at lower temperatures between 62 to 68 °C should give a tetranitrate.
The mixture is than cooled to room temperature, 1000 ml of cold water is stirred in and the result is filtered and washed with several small portions
of cold water.
I belive it will work when the benzophenol is dissolved in the H2SO4 and HNO3 will be added to this. A possible way is when benzophenone is nitrated
by step 1. or 2. and this will give dipicric acid by NaOH and MeOH .
I guess many interested explosives or similar methods of nucleophilic substitution will have a way that used dipicric acid. I`m looking for a simple
way to obtain DPA by picric acid or other ways. Anyone have some thoughts to that ?
[Edited on 11-7-2006 by Mason_Grand_ANNdrews]
[Edited on 13-7-2006 by Mason_Grand_ANNdrews]
|
|
|
YT2095
International Hazard
    
Posts: 1091
Registered: 31-5-2003
Location: Just left of Europe and down a bit.
Member Is Offline
Mood: within Nominal Parameters
|
|
what color is Di-picric acid?
and is it any good as a Dye?
Explosives I can make IF I wanted to, but I have no desire or use for any. this is only Posted in the Energetic materials thread because that`s what
it`s better known for, and I stand a better chance of a good workable synth.
\"In a world full of wonders mankind has managed to invent boredom\" - Death
Twinkies don\'t have a shelf life. They have a half-life! -Caine (a friend of mine)
|
|
|
Swany
Hazard to Others
  
Posts: 188
Registered: 11-4-2005
Location: My happy place...
Member Is Offline
Mood: Sanguine
|
|
Di-picric acid meaning 2,4 dinitrophenol? Or, a dimer/2 TNP molecules that have been mashed togeather?
It is used in dye manufacturing, like every other nitrocompound. I do not know of its dying properties, a MSDS may help. I suggest you look into azo
and diazo compounds, perhaps aminoguanidine derivs. as well.
Everyone who frequents energetic materials tends to look elsewhere on the forum as well.
|
|
|
Mason_Grand_ANNdrews
Hazard to Self
 
Posts: 63
Registered: 19-1-2006
Location: New Berlin City !!
Member Is Offline
Mood: crabby
|
|
I guess dipicric acid is yellowish. It was a vagary  only. I belive you will found
nothing in MSDS. Exuse me, some errors was corrected. I will post something like this in energetic materials. only. I belive you will found
nothing in MSDS. Exuse me, some errors was corrected. I will post something like this in energetic materials.
CAS-Nr. of benzophenone: 5350-57-2
[Edited on 13-7-2006 by Mason_Grand_ANNdrews]
|
|
|
Mason_Grand_ANNdrews
Hazard to Self
 
Posts: 63
Registered: 19-1-2006
Location: New Berlin City !!
Member Is Offline
Mood: crabby
|
|
To the last post a small attachment which give the structure of dipicric acid or something like this what DPA is. I guess DPA will be obtained by a
nitrophenol, 3-bromophenol in concentrated H2SO4 and HNO3 can give the result in figure 2.
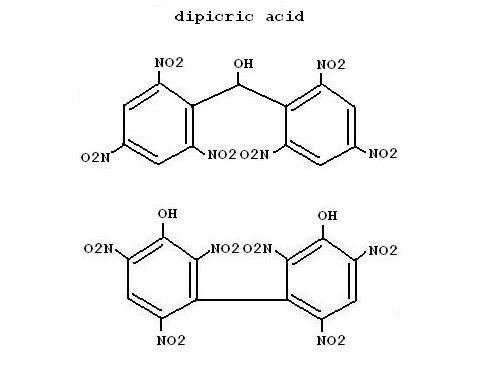
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
| Quote: | | I don't understand what you mean. Quinone is already black (well it can be anything from dark brown to dark green). In case you used the colorless
hydroquinone (since you talk about "needle like crystals" and don't mention them having a color) then it is obvious they went black as all kind of
shit happened to them. Hydroquinone easily oxidizes to quinone and other tarry crap (all black), especialy in the presence of HNO3 (to crap mostly).
|
No, what you tell here is not true.
Quinone is lemon-yellow and in fact has a really beautiful color (and a disgusting phenol-like odor).
When hydroquinone (p-dihydroxybenzene) is oxidizing in acidic media, then quinone is formed. This quinone forms a complex with remaining hydroquinone.
This complex, which has formula C6H4O2.C6H4(OH)2 is dark green. It also is called quinhydrone. Look at this site for more information on quinhydrone.
http://www.uni-regensburg.de/Fakultaeten/nat_Fak_IV/Organisc...
When the quinhydrone is further oxidized, then the solution becomes lighter again, and finally you get a beautiful deep yellow solution of quinone
(better: p-benzoquinone). On standing, crystals of quinone are separated from the liquid.
You can make quinone fairly easily yourself. I did this and I have a small sample of quinone at my home lab.
Dissolve some hydroquinone in as little as possible dilute sulphuric acid (conc. of acid is not critical).
Add a very small pinch of V2O5 (this is needed as catalyst).
Add NaClO3 (reaction takes 1 mol of NaClO3 for each 3 mols of hydroquinone), add excess NaClO3 (e.g. 1.5 times the stoichiometric amount). It probably
also works with KClO3, but the disadvantage of KClO3 is its limited solubility in water.
Shake well every few minutes.
First the solution becomes very dark (due to formation of quinhydrone). On further shaking, the solution becomes bright yellow. This reaction takes a
few hours and you need to shake a lot. It is very tedious, but the end result is very nice. You get many yellow crystals of quinone. If the liquid
becomes warm during the reaction, keep it cool with cold water from a slowly running tap.
These crystals of quinone must be rinsed with a small amount of cold distilled water and then can be extracted and recrystallized from an
organic solvent. IIRC correctly, I used diethyl ether, but a low boiling ligrion also should do the job. The resulting quinone is not really bright
yellow, it is yellow with a brown tinge, but it is good enough for my purpose and experiments.
Beware: quinone is quite toxic and it has a really horrible smell. I keep my sample in a small plastic bottle, with a larger plastic bottle around it,
and that bottle in a glass botlle. Only then I have not the horrible smell of this compound in my lab. Also, p-benzoquinone is very volatile and its
vapor stains everything with a nasty brown/red color, which cannot be removed.
=======================================================
When hydroquinone is oxidized in alkaline solution by oxygen from the air, then you indeed get all kinds of black and tarry substances. This is
totally useless crap, but it is quite interesting to see how a solution of hydroquinone in dilute NaOH quickly changes color.
Remarkably, when some sodium sulfite or sodium bisulfite is added, then the destructive oxidation by oxygen from air proceeds MUCH slower. Just try
it. Dissolve some hydroquinone in water. Make two parts of this. To one part, add a solution of NaOH. To the other part, add a solution of sodium
(metabi)sulfite and then add the NaOH and look at the difference. This stabilizing effect of sulfite is exploited in black and white photography,
where hydroquinone is used as a developer.
[Edited on 15-7-06 by woelen]
|
|
|
Mason_Grand_ANNdrews
Hazard to Self
 
Posts: 63
Registered: 19-1-2006
Location: New Berlin City !!
Member Is Offline
Mood: crabby
|
|
Why is that what i will tell not correct ? I guess there is no one way to obtain hydroxy results. I guess your link will descibes a method which the
result will decompose until one hour, perhaps it will work when the filtrate will used for a other synthesis bevor it decmpose. I think it will work
when the hydroquinone  is obtained by benzoquinone - MeOH/NaOH and the dryed
result is used for somthing more but i`m not sure if the remainder of the sodium salt in the result will be bad and this will not work by
benzoquinone. is obtained by benzoquinone - MeOH/NaOH and the dryed
result is used for somthing more but i`m not sure if the remainder of the sodium salt in the result will be bad and this will not work by
benzoquinone.
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I wrote that your remark is not correct, because hydroquinoine is not oxidized to tarry crap in acidic media and p-benzoquinone is not black/green,
but lemon-yellow.
And it works what I described, because I have a sample of the yellow p-benzoquinone. I can put a picture over here of the stuff if you like.
In alkaline solution things are totally different. You indeed get black crap. What happens in alkaline solution is that the material is oxidized and
condensed species are formed (multiple aromatic rings are connected through each other in all kinds of interconnections). The larger the condensed
species, the stronger the color. IIRC with hydroquinone the color is brown/red, with catechol the color is green. At a certain point, the condensed
species become so large that macroscopic particles are formed and that can be observed as very dark, almost black particles. These condensed partly
oxidized species are totally useless. One cannot assign a certain formula to these compounds, in fact it is a very ill-defined mix of very large
molecules. When this material is dried you obtain a dark brown powder, insoluble in water, which definitely is not p-benzoquinone, nor quinhydrone.
|
|
|
chromium
Hazard to Others
  
Posts: 284
Registered: 27-6-2005
Member Is Offline
Mood: reactive
|
|
Btw this was Nicodem who described properties of quinone incorrectly.
|
|
|
| Pages:
1
2 |