DFliyerz
Hazard to Others
  
Posts: 241
Registered: 22-12-2014
Member Is Offline
Mood: No Mood
|
|
Aluminum Chloride
So, I've been trying to deal with this for a few days, but it keeps throwing curveballs at me. I made some aluminum chloride (not very well), and it
didn't work as well as I wanted. So I decided to dispose of it by hopefully neutralizing it with sodium carbonate to aluminum carbonate (which would
decompose to aluminum hydroxide) and sodium chloride. Of course, life isn't so simple.
At first it seemed to make a sort of "shell" around the carbonate, leaving a number of blobs of sodium carbonate sitting at the bottom of my beaker. I
then put some in a flask, and added even more sodium carbonate. Fast forward a few days, with me breaking up the globs every once in a while, I now
have a very thick gelatin in my flask. How do I deal with this, and will I have to simply get someone to dispose of it for me?
|
|
|
neptunium
National Hazard
   
Posts: 990
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
how much of it do you have? if it was me ? i`d save my glassware and disolve then drain the rest of it whatever it is... why so much trouble ?
|
|
|
DFliyerz
Hazard to Others
  
Posts: 241
Registered: 22-12-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by neptunium  | | how much of it do you have? if it was me ? i`d save my glassware and disolve then drain the rest of it whatever it is... why so much trouble ?
|
Well, it's a neurotoxin and an environmental hazard, so... and it's an especially big deal because I live in an area with lots of aquifers and rivers.
[Edited on 2-25-2015 by DFliyerz]
|
|
|
neptunium
National Hazard
   
Posts: 990
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
neurotixine? where did you read that? no no no ! you have it swiming in sodium carbonate all this lewis acid is gone... get rid of it. no problem...
[Edited on 25-2-2015 by neptunium]
|
|
|
subsecret
Hazard to Others
  
Posts: 424
Registered: 8-6-2013
Location: NW SC, USA
Member Is Offline
Mood: Human Sadness - Julian Casablancas & the Voidz
|
|
Aluminum is a major component of the earth's crust, mostly in the form of oxides and basic oxides IIRC.
@neptunium: I have also heard that people who are exposed to large amounts of aluminum can develop neurological issues. I don't know the source of
this information, so nobody can really testify to it's truth. It may as well be full of BS.
This might be a roundabout way to address the problem, but what is the pH of your area's soil, DFliyers? If it's alkaline, you'll precipitate any
soluble Al compounds into the natural form.
Most likely, the only significant problem you'd have is Lewis acidity of the Al 3+ion, but that's just if you spilled it on yourself or
poured it into a potted plant.
[Edited on 25-2-2015 by Awesomeness]
Fear is what you get when caution wasn't enough.
|
|
|
neptunium
National Hazard
   
Posts: 990
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
yes i heard that aluminum is bad in the brain but you`d have to be expose to massive amount for a very long time this is just not the case here..
|
|
|
Molecular Manipulations
Hazard to Others
  
Posts: 447
Registered: 17-12-2014
Location: The Garden of Eden
Member Is Offline
Mood: High on forbidden fruit
|
|
Explain how you made this "aluminum chloride". Perhaps you mean chloride hydrate? These are two very different things. I would just throw it out, but
if you want to precipitate a less toxic form that's good.
[Edited on 25-2-2015 by Molecular Manipulations]
-The manipulator
We are all here on earth to help others; what on earth the others are here for I don't know. -W. H. Auden
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
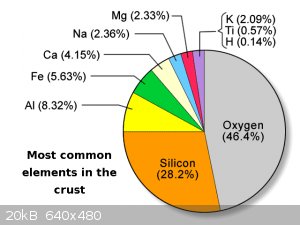
Just did a little on-line reading on aluminum toxicity: it as some interesting aspects.
As the pie-chart above shows, there is a lot of aluminum around, and so humans have always been exposed to a lot of aluminum, so the GI tract seems
efficient at rejecting/removing aluminum. But if that is bypassed the body has trouble getting rid of it (inhaling soluble aluminum compounds is
probably a bad idea)
If aluminum builds up in your body, that is not good for you.There are several targets of toxicity, the brain is one - you shouldn't have more than 2
mg of aluminum in your brain.
On the other hand, what metals can you have build up in your body without consequence?
One thing that is surprising when you think about it is that there is no essential role for aluminum. Aluminum ions can partially substitute for some
of the essential ones (not a good thing I wager), but that means there are opportunities for aluminum to replace another ion in some role - a process
that could be gradual. For a ubiquitious element, I find it odd that this apparently never happened even once.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DFliyerz  | So, I've been trying to deal with this for a few days, but it keeps throwing curveballs at me. I made some aluminum chloride (not very well), and it
didn't work as well as I wanted. So I decided to dispose of it by hopefully neutralizing it with sodium carbonate to aluminum carbonate (which would
decompose to aluminum hydroxide) and sodium chloride. Of course, life isn't so simple.
At first it seemed to make a sort of "shell" around the carbonate, leaving a number of blobs of sodium carbonate sitting at the bottom of my beaker. I
then put some in a flask, and added even more sodium carbonate. Fast forward a few days, with me breaking up the globs every once in a while, I now
have a very thick gelatin in my flask. How do I deal with this, and will I have to simply get someone to dispose of it for me? |
You've basically been using too concentrated solutions. Try dilute the Al chloride and sodium carbonate to about 1 M before mixing.
Toxicity concerns about Al(OH)3 are a nonsense.
[Edited on 25-2-2015 by blogfast25]
|
|
|