Metallus
Hazard to Others
  
Posts: 116
Registered: 16-5-2013
Member Is Offline
Mood: No Mood
|
|
dissolve corundum
I was wondering why I can readily dissolve Al2O3 (or better, Al(OH)3) in acids like HCl, but corundum won't dissolve
even in acqua regia?
Dissolving foils of aluminium in sodium hydroxide or hydrochloric acid was one of my main "activities" back then and I liked going back and forth from
the hydroxide to the chloride, seeing it precipitate and then redissolve.
I thought that corundum, which is basically Al2O3, would have behaved the same as a metal oxide, which usually exert basic
properties, and when the professor assistant told me to regenerate the corundum in acqua regia (we use corundum in a reactor as the inert phase), I
was like "Uhm, you sure?", thinking that doing so would have totally dissolved the allumina. To my amaze, it didn't dissolve even one little bit and
it was fucking concentrated HCl and HNO3 bubbling solution.
Why corundum is so resistant? The alluminium oxide/hydroxide I produced at home readily dissolved in acids, but now why the same substance won't etch
even after 2 days sitting in acqua regia? I could understand that since the particles are bigger (40-60 mesh) then the surface area exposed is less
and so it will dissolve less steadily. Yes, less steadily but here NOTHING happened at all, it only got cleaned from the little shit that was
deposited on it.
Why didn't it even get a scratch from acqua regia? What could dissolve allumina in this "form"? Why such different and counter intuitive behaviour?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Because corundum is, simply put, annealed alumina. It is much, much less soluble than most other forms of aluminium oxide or aluminium hydroxide.
Aqua Regia (AR) is a red herring here. People believe, because it dissolves gold, it must dissolve everything else too. But that's not true: AR
dissolved gold because it's a very strongly oxidising acid. But oxidising power is irrelevant here. Corundum cannot be further oxidised.
Corundum, if very finely ground, would dissolve slowly in 50 % NaOH at high temperature.
[Edited on 23-2-2015 by blogfast25]
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
It is the stability created by crystallization which makes it inert to all but the strongest attacks. Consider using carbon powder in an experiment,
compared to a diamond. Same stuff, different bond energies, different reactivity.
It turns out there really isn't much that attacks this stuff.
Check out this paper: https://nanolab.berkeley.edu/labmanual/chap1/JMEMSEtchRates2...
The scale here is nanometers per minute.
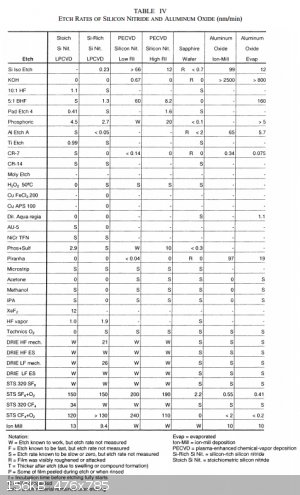
|
|
|
Caustic Window
Harmless

Posts: 2
Registered: 16-2-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by blogfast25  |
Corundum, if very finely ground, would dissolve slowly in 50 % NaOH at high temperature.
[Edited on 23-2-2015 by blogfast25] |
Are we talking molten hydroxide here? Because if anything is going to attack it, it'll be molten alkali hydroxide. If you want to go there, that is.
|
|
|
Wizzard
Hazard to Others
  
Posts: 337
Registered: 22-3-2010
Member Is Offline
Mood: No Mood
|
|
Alumina dissolves into hot molybdic acid at an appreciable rate.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Caustic Window  | | Are we talking molten hydroxide here? Because if anything is going to attack it, it'll be molten alkali hydroxide. If you want to go there, that is.
|
There's no problem 'going there' (been there, done that).
I was referring to the digestion of Bauxite ore (mineral alumina) in the Bayer process. Very finely ground ore is digested with 50 w% NaOH in
pressurised autoclaves (temp. about 200 C, I think) to yield sodium aluminate. Filtration and selective hydrolysis then separates out the silica and
ferric oxide, yielding quite pure Al(OH)<sub>3</sub>.
I believe this would work for Corundum too. Fusing with molten NaOH or KOH probably works better though.
For some ores like Beryl, fritting is used to 'soften' the closed crystalline structure of the mineral. Molten Beryl is then quenched in cold water,
which vitrifies the material. The vitrified frit is much more responsive to strong acids like conc. H2SO4 than the raw Beryl.
[Edited on 23-2-2015 by blogfast25]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4334
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Online
Mood: Semi-victorious.
|
|
It also dissolves nicely in molten cryolite.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Hahaha. You'd still have to grind it finely I think. No sinecure either.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4334
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Online
Mood: Semi-victorious.
|
|
Quote: Originally posted by blogfast25  | Because corundum is, simply put, annealed alumina. It is much, much less soluble than most other forms of aluminium oxide or aluminium hydroxide.
Aqua Regia (AR) is a red herring here. People believe, because it dissolves gold, it must dissolve everything else too. But that's not true: AR
dissolved gold because it's a very strongly oxidising acid. |
It's equally important that the presence of chloride ion stabilizes the gold(III) ions produced by forming a complex ion. Otherwise, AR wouldn't do
any better than nitric acid at dissolving gold.
But we digress.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
DA:
Don't forget nitrosyl chloride, which makes AR a stronger oxidiser than pure nitric.
But we digress.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
'digested' ?
Hmm.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
DA:
It could be an interesting exercise to evaluate 'on paper' what the contributions of the Au<sup>3+</sup> complexation and the extra
oxidising power of NOCl are to the overall estimated ΔG of Reaction for the dissolution of gold in Aqua Regia.
[Edited on 24-2-2015 by blogfast25]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4334
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Online
Mood: Semi-victorious.
|
|
Quote: Originally posted by blogfast25  | DA:
It could be an interesting exercise to evaluate 'on paper' what the contributions of the Au<sup>3+</sup> complexation and the extra
oxidising power of NOCl are to the overall estimated ΔG of Reaction for the dissolution of gold in Aqua Regia.
[Edited on 24-2-2015 by blogfast25] |
Not really. Because Au<sup>3+</sup> + 4 Cl<sup>-</sup> --> AuCl4<sup>-</sup> has a large Keq, it
can be shown to lower the overall ΔG of the reaction.
The extra oxidizing power of NOCl, on the other hand is doubtful. If it is a stronger oxidizing agent than the nitric acid, then it is unlikely to be
formed in a very high concentration (since nitric acid is too weak of an oxidizer to make it). Dilute NOCl will have a lower chemical potential, and
thus be a weaker oxidizing agent.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
I know about the complex (auric acid, basically). Do you have a K<sub>f</sub> (complexation constant) value for it?
But it looks like you are correct: NOCl doesn't play a significant part in the dissolution of gold by Aqua Regia.
[Edited on 24-2-2015 by blogfast25]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4334
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Online
Mood: Semi-victorious.
|
|
Quote: Originally posted by blogfast25  |
I know about the complex (auric acid, basically). Do you have a K<sub>f</sub> (complexation constant) value for it?
But it looks like you are correct: NOCl doesn't play a significant part in the dissolution of gold by Aqua Regia.
|
I don't have a direct Kf, but the difference in the electrode potentials is 0.50 V, which corresponds to a difference in deltaG of -145 kJ/mol, or a
Kf of 2.3 x 10^25.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DraconicAcid  | Quote: Originally posted by blogfast25  |
I know about the complex (auric acid, basically). Do you have a K<sub>f</sub> (complexation constant) value for it?
But it looks like you are correct: NOCl doesn't play a significant part in the dissolution of gold by Aqua Regia.
|
I don't have a direct Kf, but the difference in the electrode potentials is 0.50 V, which corresponds to a difference in deltaG of -145 kJ/mol, or a
Kf of 2.3 x 10^25. |
That makes a considerable difference. Ta.
|
|
|