| Pages:
1
2 |
Actinium
Hazard to Self
 
Posts: 67
Registered: 2-9-2014
Member Is Offline
Mood: synthetic
|
|
Purifying/increasing % HCl in Hardware store Acid
I was just wondering if it was better to just gas the HCl to saturation to get the 37% or make it from scratch?
Assuming that one would want to not purchase it?
JUst curious
|
|
|
vmelkon
National Hazard
   
Posts: 669
Registered: 25-11-2011
Location: Canada
Member Is Offline
Mood: autoerotic asphyxiation
|
|
Some look a little green presumably due to Fe(2+). Some of them are clear.
If your store sells dirty stuff, then go ahead with the old NaHSO4 + NaCl and lead the HCl into fresh water.
Signature ==== Is this my youtube page? https://www.youtube.com/watch?v=tA5PYtul5aU
We must attach the electrodes of knowledge to the nipples of ignorance and give a few good jolts.
Yes my evolutionary friends. We are all homos here. |
|
|
HgDinis25
Hazard to Others
  
Posts: 439
Registered: 14-3-2014
Location: Portugal
Member Is Offline
Mood: Who drank my mercury?
|
|
It's a matter of price. Most hardware HCl is dirt cheap but either has some contamination in it or isn't concentrated enough. In my case, just buying
the HCl is much more rentable than making it from Sulfuric Acid and Chloride salts.
If you can buy 33/37% HCl but it has impurities in it, simple distillation should remove them and leave you with fairly pure Hydrochloric Acid.
On the other hand, if your store only sells diluted stuff, you may want to concentrate it. HCl and water form an azeotrope that boils at 110ºC and
leaves you with 20% concentrated stuff. If you need more concentration, adding Sulfuric Acid and bubbling the resultant HCl gas may be an option...
|
|
|
Actinium
Hazard to Self
 
Posts: 67
Registered: 2-9-2014
Member Is Offline
Mood: synthetic
|
|
Quote: Originally posted by HgDinis25  | It's a matter of price. Most hardware HCl is dirt cheap but either has some contamination in it or isn't concentrated enough. In my case, just buying
the HCl is much more rentable than making it from Sulfuric Acid and Chloride salts.
If you can buy 33/37% HCl but it has impurities in it, simple distillation should remove them and leave you with fairly pure Hydrochloric Acid.
On the other hand, if your store only sells diluted stuff, you may want to concentrate it. HCl and water form an azeotrope that boils at 110ºC and
leaves you with 20% concentrated stuff. If you need more concentration, adding Sulfuric Acid and bubbling the resultant HCl gas may be an option...
|
Just so I understand correctly, if its contaminated and I want to distill it, and say it's 31.45% HCl, will it come over at 110 C. 20% because of the
azeotrope? Then would have to re concentrate it?
Also would it be advisable to dilute it first prior to distillation or can it be done as such from the bottle? I'm just starting to work with acids
and such. thank you for your replies and patience.
-AC-
[Edited on 12-1-2015 by Actinium]
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Quote: Originally posted by Actinium  | ...
Just so I understand correctly, if its contaminated and I want to distill it, and say it's 31.45% HCl, will it come over at 110 C. 20% because of the
azeotrope? Then would have to re concentrate it?
Also would it be advisable to dilute it first prior to distillation or can it be done as such from the bottle? |
Since ~31% is above the azeotropic concentration the initial distillate will be more concentrated in HCl than your original solution, in fact
I think it will simply distill pure HCl gas (not sure about this though - I've certainly never done it).
If your only concern is too increase the strength of the acid, use the 31% HCl in your collecting flask also to bubble the HCl through it. Discontinue
when the 20% azeotrope is reached, and it starts coming over.
If you are also trying to purify the acid of any salts then collect the HCl gas in distilled water, switch flasks and bring the azeotrope over, you
will end up with pure 20% HCl, with any dissolved salts left behind.
You can then use that for collecting HCl gas in a second distillation.
|
|
|
metalresearcher
National Hazard
   
Posts: 758
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
Strange. Azeotropic HCl acid is 20% and concentrated is 37%. I thought this is the same concentration. What explains this difference and how does
industry make 37% acid ?
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Concentrated hydrochloric acid is made by gassing either water or azeotropic hydrochloric acid with hydrogen chloride to the point of saturation.
|
|
|
vmelkon
National Hazard
   
Posts: 669
Registered: 25-11-2011
Location: Canada
Member Is Offline
Mood: autoerotic asphyxiation
|
|
Quote: Originally posted by careysub  |
Since ~31% is above the azeotropic concentration the initial distillate will be more concentrated in HCl than your original solution, in fact
I think it will simply distill pure HCl gas (not sure about this though - I've certainly never done it).
|
That is correct. The vapor pressure for HCl is low, so it will gas off first and concentration of your liquid in the RBF will go down to the point of
azeotrope.
The same thing happens with ethanol water mixture. If you are above the 89% mol point (or above 95% by weight), the vapor will be high concentration
ethanol. The liquid in the RBF will go down to 89% mol (95% weight).
But I suspect that it can be more complicated than. It is highly depending on the bp various ethanol + water combinations.
[Edited on 13-1-2015 by vmelkon]
Signature ==== Is this my youtube page? https://www.youtube.com/watch?v=tA5PYtul5aU
We must attach the electrodes of knowledge to the nipples of ignorance and give a few good jolts.
Yes my evolutionary friends. We are all homos here. |
|
|
Actinium
Hazard to Self
 
Posts: 67
Registered: 2-9-2014
Member Is Offline
Mood: synthetic
|
|
so since the pressure on HCl is low and will fume off, what would be the ideal and safe way to distill this to use whatever is gassing off first to
saturate dH20 until I get to the azeotrope of 20% ?
Or is it one of those things where the HCl is going to come off and just wait for the 20% solution to come over and use that as feed stock for either
saturation of dH2O or saturation of the 20% solution it's self?
I had found a post that I can ;longer find and I know Woelen had made a few comments about such as well as saying that the residual solution is great
for cleaning glassware.
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
If you start with 31.45% and boil it, HCl(g) and a small amount of water will be given off until the solution reaches 20%, at which point the rest
will distill over at 20%.
If you start with less than 20%, mostly water with a little HCl will be given off until the solution reaches 20%, at which point the rest will distill
over at 20%.
If you want to re-saturate your distilled 20% HCl, boil the 31.45% and use the vapors to gas the clean, pre-distilled stuff back up to saturation,
which will simultaneously reduce the dirty 31.45% down to the 20% azeotrope. You can then finish distilling the dirty stuff to another container to
make more clean 20%, and the process repeats itself.
You can purify without distilling by placing an open container of the HCl in a sealed bag with another open container of dH2O. The HCl will equalize
concentration in both solutions after a few days, depending on the temperature. To calculate the final concentration, simply treat it as if you were
adding the water to the HCl.
To gas HCl into a solution efficiently, keep the solution as cold as possible, keep the gas bubbles as small as possible, and keep the bubbles in
contact with the solution as long as possible. It is ideal to use a tall, narrow container and introduce gas through a long straw to the bottom of the
vessel. The straw is shaped to blow the tiniest bubbles possible. Be careful with suckback.
[Edited on 14-1-2015 by Praxichys]
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
The name for the contraption Praxichys mentions, by the way, is a gas wash bottle.

I have one of these, but every single time I use it suckback is almost instantaneous when the gas flow starts. I've never been able to use this thing
properly. Anyone have any tips?
If, like me, you are plagued with suckback, the 'inverted funnel trick' works great:
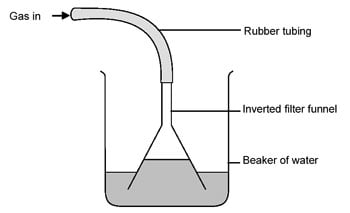
If suckback starts, the water level outside the funnel lowers enough to break the seal and stop it from flowing further up the gas line.
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Is there a better method to handle the suck back than to have a suck back flask in between the gas generator and the flask with the dispersion tube?
Is there something that handles this situation a little more elegantly? I know about one-way valves, etc., but is there cheap chemical resistant
solution for instances like this that cut off when it starts to flow the other way?
[Edited on 14-1-2015 by Loptr]
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
Quote: Originally posted by Actinium  | | I was just wondering if it was better to just gas the HCl to saturation to get the 37% or make it from scratch? |
Considering how cheap hardware store HCl is, I'd just make it from that. Why waste valuable sulfuric acid or even NaHSO4 when you can literally get a
gallon of 31.45% HCl for a few dollars? (assuming you can in your area since you mentioned it) Even if it's too impure for what ever it is you need it
for, it's still a great source of cheap HCl gas.
As others have pointed out, heating some 31.45% HCl will produce HCl gas. However, a better method--in my opinion, at least--for generating cheap HCl
gas from an aqueous solution is to simply dehydrate it using anhydrous CaCl2. Just drip the acid over some dry CaCl2 in a gas generator and you'll get
plenty of gas. This can be done with or without heat. Depending on how efficient you want to be, the resulting solution of CaCl2 and HCl can also be
distilled, and your anhydrous CaCl2 can be regenerated by heating it in the oven at 200°C for a couple of hours. (hardly worth the effort, though,
considering how dirt cheap both CaCl2 and HCl are)
If purity isn't an issue, slowly bubble your HCl gas into a cooled solution of the store-bought acid until it's saturated. If purity is what
you're after, distill over some pure 20% HCl first and then gas it. That, or use dH2O.
[Edited on 15-1-2015 by Darkstar]
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
@Loptr
Look at MrHomeScientist's post above you. That's what I use and it works wonders, the only problem is it uses a three prong clamp and ring stand.
All that is needed is a funnel and a beaker.
|
|
|
Actinium
Hazard to Self
 
Posts: 67
Registered: 2-9-2014
Member Is Offline
Mood: synthetic
|
|
thank you guys. that was really helpfull and learned alot and cant wait to give it a go. as far as safety goes, i can read msds and wiki ect.. all day
but i would like some personal tricks or things that you would only pickup on the job? are there certain materials I shouldn't use? should silicon be
used on the joints or is it like distilling chloroform where it disolves the grease into your product?
|
|
|
Zombie
Forum Hillbilly
    
Posts: 1700
Registered: 13-1-2015
Location: Florida PanHandle
Member Is Offline
Mood: I just don't know...
|
|
I've googled around for that answer, and I found several PDF's on compatibility of "Cured Silicone" or silicone "O" rings or Silicone diaphragms...
All of them indicate that Silicone (in those forms) is NOT compatible w/ HCL.
I found one post on Erowid that stated you MUST use silicone grease on joins when using HCL.
That being said I am every bit as curious as you are to here a definitive answer from several posters.
I understand set or cured silicone is different from the grease form but I would like to know how or in what way.
That is an excellent question Antinium. One I would have not thought to ask.
They tried to have me "put to sleep" so I came back to return the favor.
Zom.
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
Grease is recommended when distilling HCl since the escaping vapors can be irritating, damage polyacetal keck clips, and corrosive to anything metal
in the vicinity like rings, clamps, rods, stands.
Silicone oil is not the most inert substance around to be using in glass joints. A perfluorinated hydrocarbon grease is preferred, often sold as "high
vacuum grease" which I have found to be inert and insoluble in aqueous acids, and should be fine for your application.
You can run the distillation without grease, but be prepared to ventilate a small amount of HCl vapors that will undoubtedly escape. Use your oil not
on the joints, but instead to coat the rods and stands with a thin film to prevent attack by HCl. Adequate ventilation is a must.
To avoid grease contamination with chloroform, use only a very tiny amount of grease, or do not grease any joints beyond the still head and instead
allow the distilling liquid to penetrate and seal the joint. Some leakage will occur in the latter method; it is really a matter of choice whether
this presents a problem, depending on the adequacy of your ventilation.
|
|
|
Zombie
Forum Hillbilly
    
Posts: 1700
Registered: 13-1-2015
Location: Florida PanHandle
Member Is Offline
Mood: I just don't know...
|
|
Very helpful. I copy/pasted this from one manufacturer... About 40.00 USD for 5 oz. I imagine that is 1/2 a lifetime worth if used correctly.
http://www.2spi.com/catalog/vac/y-vac.shtml
Fomblin® VAC™ 3 is a perfluoropolymer based vacuum grease thickened with a PTFE thickener. It is an inert, homogeneous, and white grease
particularly suitable for use as a lubricant for mechanical parts or seals operating at high vacuum and in contact with aggressive chemicals or
oxygen. VAC 3 is used in lubricated "for life" applications; its lubricating properties make it suitable for parts requiring boundary (extreme
pressure) lubrication in a temperature range from -20°C to 250°C. VAC 3 is designed to meet MIL-G-27617 Type III specifications and test method ASTM
E-595-77 as tested by NASA/GSFC (extremely low outgassing in a vacuum environment). Before making any decision about your vacuum grease requirements,
be sure to be aware of some of the other information and specifications concerning VAC 3 high vacuum grease.
They tried to have me "put to sleep" so I came back to return the favor.
Zom.
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Yeah, perfluorinated grease is rather expensive, but a tube that big will last for many years. I haven't invested in one yet, but I've ruined a
multitude of Keck clips in the past few months so I probably should.
|
|
|
macckone
Dispenser of practical lab wisdom
    
Posts: 2168
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
United glass tech sells ptfe sleeves for ground glass joints.
Plumbers pfte tape also works well.
|
|
|
Zombie
Forum Hillbilly
    
Posts: 1700
Registered: 13-1-2015
Location: Florida PanHandle
Member Is Offline
Mood: I just don't know...
|
|
I've seen those rings, and read about the PTFE tape method.
Both are less expensive but IMHO they could be more problematic in the long run. I'm a K.I.S.S. kinda guy
They tried to have me "put to sleep" so I came back to return the favor.
Zom.
|
|
|
Actinium
Hazard to Self
 
Posts: 67
Registered: 2-9-2014
Member Is Offline
Mood: synthetic
|
|
that being said, I really need to invest in building a Fume hood, and getting some More protective gear for my face ect... I imagine is a must..
I'll get to it and report my results.
And will vinyl tubing work or do we need something steadier?
|
|
|
Zombie
Forum Hillbilly
    
Posts: 1700
Registered: 13-1-2015
Location: Florida PanHandle
Member Is Offline
Mood: I just don't know...
|
|
For supporting a fume hood? (vinyl tubing)
I'd think more like threaded rod, and covered w/ vinyl shrink tubing. Just one approach.
I mounted mine under a shelf, and vented it straight out the back thru the outside wall.
They tried to have me "put to sleep" so I came back to return the favor.
Zom.
|
|
|
Sniffity
Hazard to Self
 
Posts: 70
Registered: 27-12-2014
Member Is Offline
Mood: No Mood
|
|
The Home Scientist (Robert Bruce Thompson) has a fairly simple method for purifying OTC HCl: https://www.youtube.com/watch?v=jv1Ms6Subg4
Question for everyone, though: Is there any way to confirm that hardware store HCl has impurities dissolved in it? What sort of impurities are we
likely to find in hardware store HCl, and why?
|
|
|
Zombie
Forum Hillbilly
    
Posts: 1700
Registered: 13-1-2015
Location: Florida PanHandle
Member Is Offline
Mood: I just don't know...
|
|
I copy/pasted this...
" Pure grades of hydrochloric acid are colorless, but technical grades, commonly called muriatic acid, are often yellow-colored because of impurities
such as dissolved metals. Most hydrochloric acid produced has a concentration of 30% to 35% hydrogen chloride by weight."
The entire article is here...
http://encyclopedia2.thefreedictionary.com/Anhydrous+hydroch...
Although it does not detail the exact "metals"/salts, it does give enough info to know what you are using.
I linked the same vid as you in another thread, and was assured that is a good method for purifying store bought Muriatic acid, and there is some
information for concentrating the resulting "reagent grade HCL. Here is that thread... http://www.sciencemadness.org/talk/viewthread.php?tid=17638&...
They tried to have me "put to sleep" so I came back to return the favor.
Zom.
|
|
|
| Pages:
1
2 |