| Pages:
1
2 |
leopard
Harmless

Posts: 12
Registered: 14-11-2002
Member Is Offline
Mood: No Mood
|
|
Dinitrourea
Rosco Bodine provided a copy of a paper wherein the authors simply state that approx. 11 ml of H2SO4 and aprox. 13 ml of HNO3 are mixed and cooled to
0 deg. C then 3 grams of urea is added over 1/2 hour while keeping the mix below 5 Deg. C and the mix is allowed to sit for another 1/2 hour at 0 deg
C. The author states that 5 grams of very pure Dinitrourea precipitated out. I attempted to follow these instructions as closely as possible using 93%
H2SO4 drain cleaner and HNO3 of unknown concentration but I made it from 93% H2SO4 and KNO3 so it should be reasonably strong. My HNO3 is quite
yellow. Here is what I did...
- mixed 11 ml H2SO4 & 13 ml HNO3 and chilled to 0 deg. C
- slowly added 3 grams powdered urea over 1/2 hour keeping the temp at 0 deg. C with constant stirring
- set the mix aside for 1/2 hour while mainting 0 deg. C
While adding the Urea I noticed the mixture became milky while stirring but rapidly became clear when I stopped stirring. When I finished adding the
Urea and set the mix aside the mixture became clear (with yellow tint from yellow acid) and no precipitate formed at all. I am wondering if it did not
work because my acids where not pure or possibly because I was not able to measure acid volumes to 1/10th ml like the authors of the article did. The
process they describe seems so simple that it would not seem possible to fail. Has anyone else tried this synth or have any ideas?
EbC:title
[Edited on 18-6-2006 by chemoleo]
|
|
|
Maja
Hazard to Others
  
Posts: 143
Registered: 27-2-2006
Member Is Offline
Mood: No Mood
|
|
I think thats due water in your acids.dinitrourea rapidly hydrolyses in presence of water it's highly unstable in water.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Random thought
This seems so obvious it must have occurred to someone.
Dinitrourea is strongly acidic to the point that it soon becomes hydrolyzed.
It's esterfication with Ethylene Glycol bears structural relation to Keto RDX
and this will yield 14 moles of gas per mole.
Note :
on page 394 of COPAE it shows Dinitroethyleneurea as the precursor
"refluxed with water" to become hydrolyzed into Ethylenedinitramine.
Dinitroethyleneurea can be thought of as the ester of one mole Dinitrourea
with one mole of Ethylene Glycol, so this casts doubt on the feasibility of
a larger molecule.
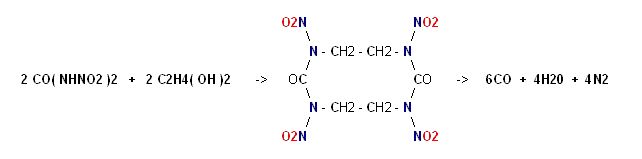
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Ummm ... that structure is not an ester, it's a urea of a diamine - ethylene diamine. No RCO2R' in it at all.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
An amide then , okay I stand corrected. It's been a long time and it shows.
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
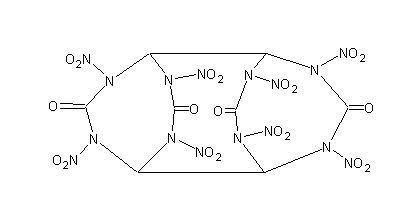
Yes, but why not use glyoxal instead of ethylene glycol? (see attached image) (see attached image)
It's like 2 diketo HMXs stuck together
And to the origional poster, a link to the paper with the procedure would be nice so we can see exactly what was done previous to your experiment.
EDIT: OOPS. how could I forget about TNGU, thanks Axt.
Ignore my picture
[Edited on 6-6-2007 by The_Davster]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Maybe this was the paper
Attachment: dinitrourea.pdf (85kB)
This file has been downloaded 1855 times
|
|
|
Axt
National Hazard
   
Posts: 794
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
Glyoxal condensation would give you tetranitroglycoluril (aka. TNGU or SORGUYL). Theres two synth's in journal <i>Propellants, Explosives,
Pyrotechnics</i>. Also one from a Russian journal as an intermediate to nitramide.
<b>PEP, 26, 17-20 (2001)</b>
95% Sulphuric acid (10.9ml, 20g, 0.19mol) was added to 100% nitric acid (13.2ml, 20.0g, 0.32mol) and then cooled to 0°C. Urea (3g, 0.05mol) was then
added to the reaction mixture for about 30 min. Care was taken that the temperature onf the reaction mixture wasn't allowed to rise above 5°C. After
the addition the reaction mixture was allowed to stand for another 30min at 0°C, during this time a white precipitate formed. The white crystal mass
was filtered off on a glass funnel and then washed with trifluoroacetic acid (5x5ml). The product was dried over vacuum giving 5.0g (0.033mol,
67mol-%) of N,N'-dinitrourea.
<b>PEP, 23, 155-158 (1998)</b>
DNU was obtained via a direct nitration of urea with a mixture of equal parts of 98% HNO3 and 20% oleum. The stirred mixture of acids was cooled at
-15°C to -10°C and urea was dosed at such rate to keep the reaction temperature below 0°C. The stirring was continued for 30 min at temperature
0°C to +5°C. The resulted precipitate of the crude DNU was ®ltered, cold and used as the starting material in further transformations. The yield of
the crude product was ca. 95%. At room temperature, particularly in the presence of water and traces of acids, DNU undergoes decomposition which may
lead to spontaneous ignition. The pure DNU has an ignition temperature 147±9°C. The identification of the DNU structure was performed after its
transformation into diammonium salt.
<b>Russian Journal of Organic Chemistry, Vol. 38, No. 1, 2002</b>
Preparation of nitramide from urea: Oleum (20% of SO3), 35 g, was added to 35 g of nitric acid (d = 1.5 g/cm3), and 10 g (0.1667 mol) of urea was
added in portions at -5 to 0°C under continuous stirring. The mixture was stirred for 40 min at 0 to 5°C and poured while stirring into 300 g of an
ice-water mixture, maintaining the temperature below 10°C. The mixture was extracted with ethyl acetate (2x100 ml and 4x50 ml), and the extract was
washed with water (3x40 ml), kept for 2 h at 20°C, and evaporated to dryness under reduced pressure. Yield 15.5 g (75%). The product was dissolved in
30 ml of ether, the solution was poured into 400 ml of hexane, and the precipitate was filtered off. Additional recrystallization from
dichloroethane-2-propanol (9 : 1) gave 13 g (63%) of nitramide with mp 78°C.
Preparation of nitramide from dinitrourea: The nitration of urea was carried out as described above. After keeping for 40 min at 0-5°C, the mixture
was cooled to -12°C, and the precipitate of dinitrourea was filtered off through a glass filter, washed with cold methylene chloride (3x10 ml), and
squeezed. The product was dissolved in 300 ml of ether at 0°C under continuous stirring, and the solution was washed with water (2x20 ml), kept for
4.5 h at 20°C, and evaporated to dryness under reduced pressure. Yield 15 g (73%).
[Edited on 8-6-2007 by Axt]
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
How's about condensing it with perbromoethane or so, and, um, hmm those amines will need to be quaternary (which would be quite a feat with the nearby
nitro groups, huh?)... Would be fun to do if it could be done though! 
Can you turn urea's amines into quaternary units then add nitro groups? Ehwtf, hell, just stick with nitrate, and balance the charge... Almost a
cryptand thing going, but not nitratey enough...maybe the side chains could be nitrated. And those electronegative carbonyls are annoying...they'd be
better as...like...gem-nitro or something 
Yeah yeah, I'm going to bed now :rolleyes:
Tim
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Things like DNU, tengu, k6, c20, medina etc all require anhydrous HNO3 and Ac20 or P2O5 to obtain any decent yields, which pretty much limits their
feasability for amateur synthesis.
So in the perspective of creating an OTC high density nitramine, what would happen if you would bubble phosgene trough a solution of EDNA in ether, or
probably even better, some metal salt of EDNA?
Axt has shown that one of the few nitramines that can be produced in high yields from mixed acids OTC is EDNA. Although incredibly dangerous,
phosgene can be produced by heating CCl4 or HCCl3(not sure) with conc. sulfuric acid.So taken that tetra is still OTC in many places this would
defenitely be possible. The reaction of phosgene with EDNA or its salts under anhydrous conditions might produce
2-oxo-1,3-dinitro-1,3-diazacyclopentane or preferably 2,7-dioxo-1,3,6,8-tetranitro-1,3,6,8-tetrazacyclodecane (from Franklyns picture). Both would be
more dense than RDX and the latter might even be denser than HMX. 
[Edited on by nitro-genes]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
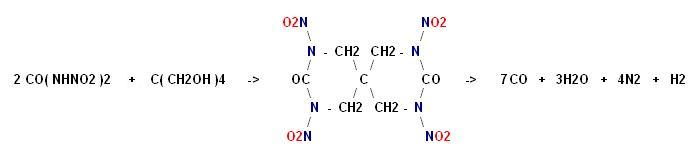
I don't know what became of the rest of the text that was here about condensing pentaerythrite with dinitourea.
Click for full size
http://img45.imagevenue.com/img.php?image=96155_x_122_626lo....

Argus Lab file here _
http://www.keepmyfile.com/download/cd1de51691521
[Edited on 26-6-2007 by franklyn]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
3 mols of Dinitrourea can also be condensed with 2 mols of Triaminobenzene _
http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=6695...
http://cdb.ics.uci.edu/CHEMDB/Web/cgibin/ChemicalDetailWeb.p...
yielding Tri-dinitrourea Di-triaminobenzene
.
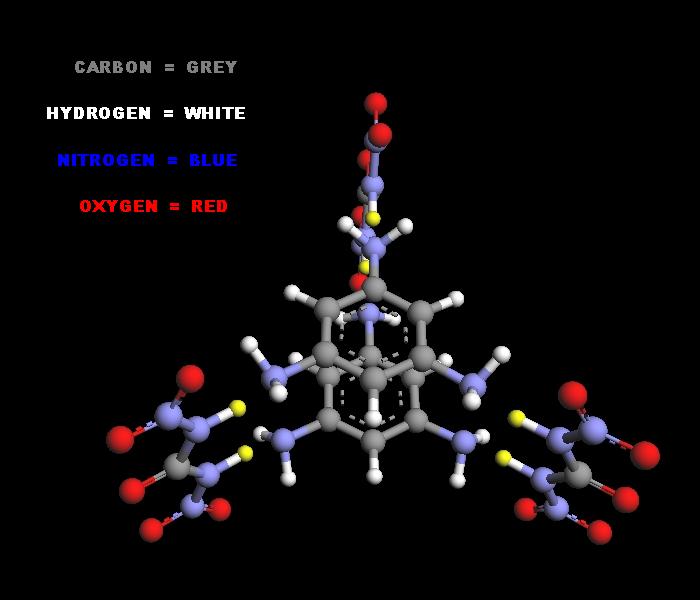
|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
Why such difficulties are needed, it's far more simple to prepare nitramide by action of HNO3 on potassium sulfamate:
NH2SO3K + HNO3 => NH2NO2 + KHSO4
|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
| Quote: | <b>Russian Journal of Organic Chemistry, Vol. 38, No. 1, 2002</b>
Preparation of nitramide from urea: Oleum (20% of SO3), 35 g, was added to 35 g of nitric acid (d = 1.5 g/cm3), and 10 g (0.1667 mol) of urea was
added in portions at -5 to 0°C under continuous stirring. The mixture was stirred for 40 min at 0 to 5°C and poured while stirring into 300 g of an
ice-water mixture, maintaining the temperature below 10°C. The mixture was extracted with ethyl acetate (2x100 ml and 4x50 ml), and the extract was
washed with water (3x40 ml), kept for 2 h at 20°C, and evaporated to dryness under reduced pressure. Yield 15.5 g (75%). The product was dissolved in
30 ml of ether, the solution was poured into 400 ml of hexane, and the precipitate was filtered off. Additional recrystallization from
dichloroethane-2-propanol (9 : 1) gave 13 g (63%) of nitramide with mp 78°C.
[Edited on 8-6-2007 by Axt] |
I've tried this method. 21 ml of commercial (93.5%, density 1.83) H2SO4 is mixed with 26 ml home made concentrated HNO3 (density 1.479 ~ 88%conc). To
remove water 35g of P2O5 was added slowly with cooling and stirring. Acid mixture was cooled on ice/salt bath to -15C, after this additon of urea
started. Reaction is EXTREMELY virgous, even smallest drops with very fast efficent stirring resulted in sudden strong heat evolution, accompanied by
evolution of white gas (like smoke) without odor (probably N2O). I've tried all stirring speeds from hand stirring to mechanical via motor, tried to
add smaller ammounts of urea, nothing seems to stop this extremely violent process. For example addion of 0.05g of urea resulted temperature rise from
-15 to +10 withing 5 seconds. I don't know precise reason of this, may be acids are too concentrated.
Since after some urea added reaction became much less violent, but still highly energetic, i concluded that the reason is presence of dissolved
nitrogen oxides. Be aware, don't try to produce dinitrourea if acid contain NOx, this makes process extremely dangerous! Anyway, i continued reaction
to the letter as in text, and got snow white precipitate of dinitrourea with 50% yield. Photo of product under mother liquer is attached to the
message:
[Edited on 4-10-2007 by Engager]

|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
Reaciton scheme is shown below. Water at low temperature hydrolises dinitrourea to form CO2 and nitramide. Nitramide is also unstable and decomposes
slowly to form N2O and water.
[Edited on 5-10-2007 by Engager]
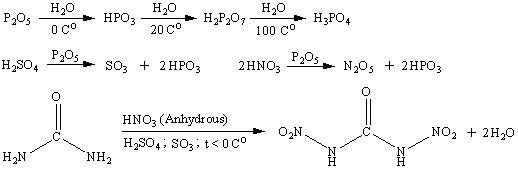
|
|
|
Axt
National Hazard
   
Posts: 794
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Engager
nothing seems to stop this extremely violent process |
I imagine the neutralisation of urea at those concentration would be quite hot. Why not try adding urea nitrate instead to see if the violent reaction
still occurs?
[Edited on 4-10-2007 by Axt]
|
|
|
simply RED
Hazard to Others
  
Posts: 209
Registered: 18-8-2005
Location: noitacoL
Member Is Offline
Mood: booM
|
|
Engager, when did the precipitate form?
When logic and proportion have fallen sloppy dead...
|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
| Quote: | Originally posted by simply RED
Engager, when did the precipitate form? |
After urea addition is completed, mixture is left to stand for 2-3 hours in ice bath. I just placed mixture in ice bath and gone for a walk, i
returned about 2-3 hours after, and mixture was full of snowy white precipitate.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
nitramide from chloramine and nitrite
It occured to me if gassing sodium nitrite with chloramine has been considered as a
way to form nitramide : H2NCl + NaNO2 -> NaCl + H2NNO2
UPDATE
Upon further reading according to Wikipedia unsolvated chloramine is unstable
and it can be inferred it is only stable in liquid form in the narrow range between
its freezing temperature -66º C and -40º C
Alternatively then liquified H2NCl could be applied guardedly in this cold fluid range
although there seems that there is a marked propensity for explosion if the materials
react too vigorously or even hypergol.
The one pot synthesis with OTC reagents is too attractive to dismiss lightly.
Buffering with a suitable chlorinated organic solvent is a logical avenue for
investigation.
UPDATE 2
CAS 10599-90-3 - From the CRC Handbook of Chemistry and Physics 85th ed
H2NCl , Chloramine is soluble in H2O , Ethanol , Diethyl ether
slightly in Benzene and Tetrachloromethane
Brauer's page 477 details the preparation of monochloramine
garage chemist noted about aqueous chloramine
" Equimolar solutions of ammonia and faintly alkaline hyporchlorite will go to chloramine
rapidly and almost quantativly in the cold. Its stable for fairly long periods."
Notes on formation for water sanitation
" Monochloramine , Dichloramine , and Nitrogen Trichloride mix of species produced
depends on the ratio of chlorine to ammonia and the pH of the water. In the pH range
of 7 to 8 with a chlorine to ammonia weight ratio of 3 to 1 monchloramine is the
principal product. At higher chlorine to ammonia ratios or at lower pH values ( 5 to 7 )
some dichloramine wil be formed , equal amounts at 5.5 . If pH drops below 5 some
Nitrogen Trichloride may be formed."
Anhydrous chloramine produced in the liquid phase
http://stinet.dtic.mil/oai/oai?verb=getRecord&metadataPr...
HCl(gas) + NaN02(solid) -> NaCl(so1id) + HONO(gas)
Environmental Science and Technology Num. 29, (1995).
A. Febo, C. Perrino, M. Gherardi, R. Sparapani. pages 2390-2395
This last cited reference evokes the possibility of reacting nitrous acid gas
directly with chloramine in the gas phase to obtain nitramide.
HONO + H2NCl -> HCl + H2NNO2
Thus anhydrous solvation is obviated , introducing instead HCl self regenerated
catalyst.
.
|
|
|
Plasmapyrobattics
Harmless

Posts: 10
Registered: 29-12-2008
Member Is Offline
Mood: Plasma...
|
|
This is interesting.
Is there any further information available on this condensation product with dinitrourea and pentaerythritol ? Synth procedure? Name? Properties?
Franklyn? Anyone?
Thanks
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
@ Plasmapyrobattics
| Quote: | Is there any further information available on this condensation product
with dinitrourea and pentaerythritol ? Synth procedure? Name? Properties? |
- No
This is a random speculation by a non-chemist , me.
Sometimes things are not so straight forward as they
may seem. The problem as I see it is the instability
and hydrolysis of dinitrourea in a procedure that
makes water as a by product.
Perhaps a salt of dinitrourea and a halo-pentaerythrite
may be induced to double replacement , I don't know
.
Techniques to Disrupt, Deviate and Seize Control of
an Internet Forum In case you wonder W T F ! is going on here
?
www.zerohedge.com/contributed/2012-10-28/cointelpro-techniques-dilution-misdirection-and-control-internet-forum https://web.archive.org/web/20120814124000/www.washingtonsblog.com/2012/08/the-15-rules-of-internet-disinformation.html
___________________________________________________________________________________________________________________
___________________________________________________________________________________________________________________
___________________________________________________________________________________________________________________
|
|
|
maxidastier
Hazard to Others
  
Posts: 118
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Can anyone explain why they use trifluoroacetic acid (5x5ml) to wash the dinitrourea?
It's pretty expensive...
Are there other ways?
|
|
|
Perihelion
Harmless

Posts: 1
Registered: 4-8-2011
Member Is Offline
Mood: No Mood
|
|
I followed the same procedure and got nothing..  and TFA is used to remove acidic
contents to make it stable over room temperature.... Is there abny other method to synthesize DNU? and TFA is used to remove acidic
contents to make it stable over room temperature.... Is there abny other method to synthesize DNU?
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by franklyn  | It occured to me if gassing sodium nitrite with chloramine has been considered as a
way to form nitramide : H2NCl + NaNO2 -> NaCl + H2NNO2
|
There could not be any water in the reaction, otherwise the chloramine would just oxidize the sodium nitrite. DMSO might make a good solvent
(surprisinly it is sold in my local health food store  , although it is rather
expensive) , although it is rather
expensive)
In dilute solutions, chloramine has some equilibrium:
NH2Cl + H2O <==> NH3 + HOCl
(at higher solution concentrations, chloramine tends to decompose releasing nitrogen)
Dinitroguanidine also exists.
Dinitroguanidine is the product of nitration of nitroguanidine. The reaction rate is much faster with a 2% solution of N2O5 in HNO3. Dinitroguanidine
dissolved in water solution, slowly hydrolyzes after prolonged standing to precipitate nitroguanidine. It is moderately soluble in both water and
organic solvents and the solubility quickly increases on warming. At 20C its solubility in water is 53g/L, in ethyl acetate 86g/L. Dinitroguanidine
can be recrystallized form alcohol or acetic acid, being a sufficiently stable compound with a melting point of 169C. Nitroguanidine forms salts with
alkali dissolved in alcohal. Under sufficient acidic conditions, Dinitroguanidine hydrolyzes to nitroguanidine, while under only moderately acidic
conditions nitrourea is formed.
Russian Journal of Organic Chem, Vol39, #4, p501
"Synthesis and some Properties of 1,2-Dintroguanidine"
A.A. Astrat’yev, D.V. Dashko
State Technological Institute,
St. Petersburg, Russia
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
I personally would start by producing urea nitrate to make the reaction more controllable. Plus, less nitric acid would have to be used, because it
already has some in the molecule. I dream about making TNGU, but maybe in a far future, if ever.
Rest In Pieces!
|
|
|
| Pages:
1
2 |