| Pages:
1
2
3 |
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
Storing sodium
I finally got my hands on a few dozen grams of Na-metal and I want to know how best to store it for future use.
Ideally I would store this stuff under a vacuum in a sealed ampoule. Not having a strong vacuum pump or a sufficiently large ampoule, this is not an
option for me.
The method I’ve seen most is storing it under mineral oil. I tried that but it always develops a pesky oxide layer after a week or two. Some oxygen
will always dissolve in the oil and get to the Na, eventually forming a white crust of sodium (hydr)oxide.
I tried storing it under paraffin wax but this didn’t work out so well. When the wax solidifies (after I add the Na) it always contracts and pulls
away from the side of the container. This allows air to come very close to the sodium and gives it a short path to travel before it hits the metal
and, once again, oxidizes it.
Would Vaseline work any better? I know that it doesn’t pull away from container walls when contracting during solidification. It’s not a liquid,
so oxygen shouldn’t diffuse into it as quickly. Right?
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
I have seen very very old alkalis in various storage mediums thanks to a new contact of mine.
When I got my sodium, it had been in a lab occasionally used for 11 years maximum. It was under parafin oil (not wax), a liquid less viscous than
mineral oil. I transfered it to a jar of mineral oil a while ago, the oxidation layer does not seem much thicker if at all, but only time can tell.
I also attempted to get some lithium, but the bottle was ancient and over time the parafin oil had evaporated(cap was leaky) leaving the top 4 cm of
the lithium sticks exposed. An entire 100g bottle of lithium rods was mostly lithium hydroxide with perhaps 5g of lithium metal left.
(sorry ChrisTG, I tried)
I have seen sodium stored under benzene, big brick sized lumps of sodium with an oxide layer which looks really thick. Oxygen must dissolve in
benzene apreciably.
I attempted obtaining potassium as well, however the can available was really, really old, most of the K must have reacted making KOH which was eating
away at the can. It looked like it had tumors. Due to potential superoxide formation I left this can alone.
I would say that tightly sealed containers of alkalis under mineral oil are the best, any oxygen in the container will react with the sodium making
the oxide layer, with a tightly sealed container hopefully no more oxygen can get into react. Just keep the container closed and minimize how much
you play with the contents . .
I would only go with vaccuum ampoules if you want to keep it shiny. Interestingly enough, my lab job has taught me how to make vaccuum ampoules by
use of 2 sizes of glass tube, oxy hydrogen torch and a vaccuum pump. Learning to work with glass takes a lot of patience (and burns)
[Edited on 15-5-2006 by rogue chemist]
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
I have some 2 years old sodium which is still fresh and with minimal oxide layer (less than a mm thick).
It is stored under low- viscosity paraffin oil, in a glass bottle with screw cap.
The trick is to fill the container nearly completely full with the oil, so that only a very small airspace is in there.
That way only a small amount of oxygen can get into it each time the container is opened, and it keeps much better that way.
We have some sodium in school, in a halfway- oilfilled bottle and it has turned mostly into oxide.
Filling the container completely with oil is most important!
Also, the cap must seal tightly.
If those precautions are met, sodium can be stored for many years with minimal loss, even with frequent use.
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
Just keep it under kerosene or mineral oil in a tightly sealed bottle, just as everyone else has recommended. I've seen pounds of it stored in the
stock room that's over 40 years old, and it's still in good condition. So don't worry about it oxidizing to dust. A couple months ago, I opened a 22
year old 100g can of it (rusted completely) to find a white brick  After
inspection with a large, flat-bladed spatula, I realized that the oxide was only 1/4" thick, underneath that was the whitish/gold sodium. Despite how
soft it was, it was still a pain to cut. Once you warm some up in your gloved hand, it's kind of like cold butter. After
inspection with a large, flat-bladed spatula, I realized that the oxide was only 1/4" thick, underneath that was the whitish/gold sodium. Despite how
soft it was, it was still a pain to cut. Once you warm some up in your gloved hand, it's kind of like cold butter.
|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
Could you post a detailed example of using the 2 size glass tubes to seal an ampoule?
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
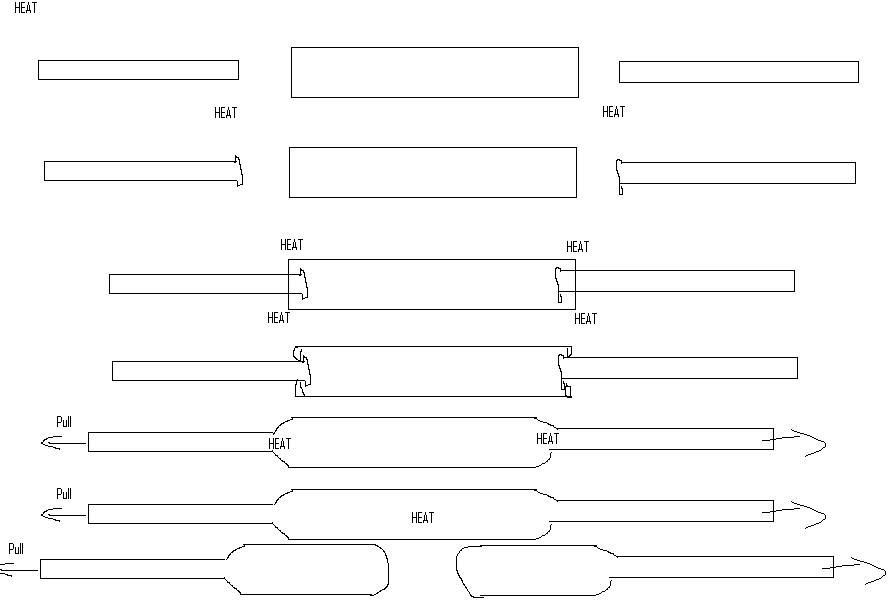
1)get 2 small diameter glass tubes, these will be the neck of the ampoule, choose size accordingly. Also get a larger diameter glass tube for the
main ampoule body
2) Using a graphite rod for manipulation and a very hot torch(I use oxy-hydrogen) and wearing special goggles, glass gets white hot, play the hot ends
of the small diameter glass tubes into flared ends. Symetry good here.
3) Insert flared small glas tube into larger diameter glass tube. Heat large glass tube on edge and using the graphite rod while rotating the tube
assembly push the heated outer glass onto the inner flare.
4) While rotating the tube with the flame on the joint gently pull the tubes in oposite directions. Play the glass with graphite rod if you gotta
fill little holes. 3 hands would be nice here, I usually rotate with my left hand, steady other side and pull with right pinky while holding the
graphite with the first 3 fingers of my right hand.
5) Repeat for other small flared tube and oposite side of large tube.
6) once satisfied that you have no holes(vaccuum pump makes funny sounds later if they arent sealed) heat the centre of the tube while rotating. Draw
out glass when soft. Use graphite to get nice ends on ampoule bottoms.
7) Ampoules get filled, attached to the vaccuum line, evacuated for 30 min, and under vaccuum the neck of the ampoule is heated while
rotating(otherwise vaccuum sucks glass in and can pop the tube there) and carefully drawn out/sealed.
Wear gloves when beginning! Worst part is flaring the small tubes, heat goes through tube and out other end, right where your gloved hand is. With
non-dry tubes steam comes out which gets painful.
And a paint diagram:
|
|
|
mick
Hazard to Others
  
Posts: 338
Registered: 3-10-2003
Member Is Offline
Mood: No Mood
|
|
To store the sodium, you could pre-treat the mineral oil like clarifing butter. Warm it up slowly to get rid of any water and air.
mick
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
I pre-treated it by adding a little sodium beforehand. The oil was very clean and reacted little with the metal.
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
Update: I checked on the metal today. There was still no air present in the bottle and the sodium was still relatively shiny.
This storage method is definitely working but it has some drawbacks. To get small amounts of Na out, you must cast it as small globules before putting
it in the bottle. This is long, tedious, and exposes a huge surface for oxidation. You can only really get the pieces in and out if you use a
wide-mouthed bottle and large tweezers.
To close the bottle, you need to top off the oil, screw the lid on, and hope there’s no air bubble in the bottle. This always causes some oil to
spill, making this process messy and a general pain. Don't even think about putting labels on these bottles because they will get oily fast.
I have a new idea, but I'm not sure if it's worth the extra effort.
First, start with a suitably sized syringe. Add some mineral oil and remove the air bubbles. Then, simply suck up your sodium into the syringe. When
you’re done, plug the end.
This way, oxygen has only two ways to get to the sodium. The first is between the rubber septum and the plastic wall of the syringe. This is supposed
to be a tight fit, so I doubt much diffusion will happen here. Just to be safe, you could fill the air space between the septum and the plastic wall
(a ring of air, several mL) with vaseline. This would harden on cooling, impeding air molecules. (Wax wouldn’t work because it contracts too much on
cooling. Plain mineral oil might also do the job.)
The second way in is through the exit point of the syringe. This is only a couple of mm<sup>2</sup> and can easily be plugged.
This would have its own ritual to extract the sodium, of course. First the whole mess would need to be melted with a steam bath or maybe a blow dryer.
The tip would be plugged to keep air out and the piston would be allowed to move freely to accommodate the volume changes that occur from melting.
Next, the desired amount of liquid metal could be squeezed out, the tip replugged, and the whole thing be allowed to cool down. This way oxidation is
kept to an absolute minimum without an inert atmosphere. 
The main problem here is the rubber in the septum. I don’t know what these are usually made of. Some rubbers (e.g. neoprene) contain chlorine,
others sulfur, but I have no idea what kind they use in syringes. Does anyone know?
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
I've got to imagine any rubber would be permeable, although, certain rubbers are used for vacuum apparatus, so there must be suitable ones.
I'm having bad images of a tube of silicone caulk that I discovered the other week. I cut open the end and gave the gun a squeeze, nothing. What the
heck. I cut the casing open, turns out the remaining product was a white cylinder of rubber! The very center was still a little gooey, though
useless.
Tim
|
|
|
Jdurg
Hazard to Others
  
Posts: 220
Registered: 10-6-2006
Location: Connecticut, USA
Member Is Offline
Mood: No Mood
|
|
Storing alkali metals is a royal pain in the ass. With Cesium and Rubidium, at least you have but one option and that's sealing the metals inside a
glass ampoule. (My Rb and Cs are still bright and shiney to this day.  ).
But for Li, Na, and K you just need to accept "some" loss due to oxidation. ).
But for Li, Na, and K you just need to accept "some" loss due to oxidation.
Lithium, in particular, is the most difficult because of the fact that it not only reacts with oxygen, but with nitrogen as well at room temperature.
Therefore, it will react with 90+ percent of the composition of the atmosphere. In addition, it is so incredibly not dense that it will float in
typical mineral oil.
What I've done to store my alkali metals is a multi-step process. The first thing I did was purchase some jars with a screw-top cap that could be
vacuum sealed if one wanted to. (I just don't have the apparatus needed to seal the jars). The jars were thoroughly cleaned and some Teflon Tape was
wrapped around the threads of the jars to ensure a tight fit was made. I then went and heated the mineral oil that I had until no more gas bubbles
were forming and escaping. The oil was quickly placed into a bottle and the top put on it to prevent any gases from seeping back in.
At this point in time I took the alkali metals I had and scraped off some oxidation to leave one side bright and shiney and the rest quite oxidized.
(As a way to show the oxidation of the metals). The sodium was just a bunch of chunks because I bought two ounces of it and they came in tiny little
lumps. Some of it was cut, but most of it was just put right in. The jars were filled half-way with my still warm mineral oil. Then the metals were
placed in there and the jars topped off with mineral oil. The tops were screwed on VERY tightly, then more Teflon Tape was wrapped around the outside
sealing the area where the cap met the jar. I then wrapped some electrical tape on top of the Teflon Tape to provide yet another seal.
All of this was done about 5 or 6 months ago and the bright surfaces are as bright as they've ever been. On the potassium, however, something odd has
begun to happen. I had excised some possible peroxide contamination on a freshly cut surface leaving a few areas that were pure metal; no oxidation.
There areas where pure metal was showing have taken on a metallic, blood red color. The sheen is VERY metallic, but the color is a deep, dark red.
This does not appear to be any type of peroxide/superoxide on the surface as those are generally yellow in color. I think what's happening is that
any peroxide/superoxide on the crust of the potassium has begun to decay due to the lack of oxygen in the surrounding oil. This oxygen is then slowly
reacting with the exposed metal surface forming an anondized layer of potassium oxide on the fresh metal surface. As K2O is far more stable than K2O2
or KO2, this layer will remain stable for a long, long time. Looking at all parts of this potassium chunk, I see little red dots in the pits of the
oxide crust. I truly think this is being caused by slow decomposition of the peroxides/superoxides on the metal surface. It's actually really neat
to see. I'm tempted to open up the jar and cut off some more fresh metal surface then re-seal again, but it's a lot of work to seal it up like I have
it now. 
\"A real fart is beefy, has a density greater than or equal to the air surrounding it, consists of the unmistakable scent of broccoli, and usually
requires wiping afterwards.\"
http://maddox.xmission.com. |
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Hmm interesting. Is it turning other colors in other spots? Red sounds like an interference color to me (K2O is white, right?).
Tim
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Jdurg, could you make some macro pictures of this and post them here? It sounds very interesting. It reminds me of the niobium experiment I did, which
could obtain blue and yellow colors, due to anodizing.
|
|
|
Jdurg
Hazard to Others
  
Posts: 220
Registered: 10-6-2006
Location: Connecticut, USA
Member Is Offline
Mood: No Mood
|
|
@woelen: I'll definitely do that. Just give me a bit of time to go and get a good setup.
@12AX7: The only color there is the blood red color. K2O is indeed a bright white, though initially it is a purplish color. Once I get the images
up you'll see what I'm talking about.
\"A real fart is beefy, has a density greater than or equal to the air surrounding it, consists of the unmistakable scent of broccoli, and usually
requires wiping afterwards.\"
http://maddox.xmission.com. |
|
|
Jdurg
Hazard to Others
  
Posts: 220
Registered: 10-6-2006
Location: Connecticut, USA
Member Is Offline
Mood: No Mood
|
|
Okay, below is as big a macro as I can get right now. The battery on my camera is just about to die so it has a good deal of trouble trying to zoom
in. Hopefully there will be enough detail. My apologies for the poor quality of the photo. It's currently 6:30 at night and the sun is at a level
which pretty much ruins any photography attempt while increasing the temperature in my abode by a great deal. (My Ga sample is liquid right now, so
it's pretty warm in here).
I can see some brownish discoloration on areas of the potassium, and that appears to be some minor peroxide/superoxide formation. However it's not
enough to cause me any concern. In reality, the amount is slowly going away which seems to confirm my initial belief that the unstable oxides are
decomposing and a very thin oxide layer is forming on the bare metal spots.

\"A real fart is beefy, has a density greater than or equal to the air surrounding it, consists of the unmistakable scent of broccoli, and usually
requires wiping afterwards.\"
http://maddox.xmission.com. |
|
|
Nerro
National Hazard
   
Posts: 596
Registered: 29-9-2004
Location: Netherlands
Member Is Offline
Mood: Whatever...
|
|
I've looked it up and KO2 is a yellow solid, not bloodred like this.
Might it be some sub-oxide? I knwo that suboxides of Cs exist. Maybe this also goes for K...
#261501 +(11351)- [X]
the \"bishop\" came to our church today
he was a fucken impostor
never once moved diagonally
courtesy of bash
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Oooh, that's *weird*!
Any chance it's something in the oil? Doubt it, but...
Tim
|
|
|
Jdurg
Hazard to Others
  
Posts: 220
Registered: 10-6-2006
Location: Connecticut, USA
Member Is Offline
Mood: No Mood
|
|
I'm pretty certain that it's not the oil. I've had this lump of potassium for quite some time now. I originally got it and had it in some cheap,
unboiled mineral oil and the side of the lump that is shown in the photo above was freshly cut. All around the rest of the lump was an oxide crust
which had the occasional spot of yellow/orange on it. I kept it in a simple screw-top jar with mineral oil in there, but no Teflon tape seal. Over
time, it slowly oxidized and the bright metal finish took on the classic purple haze of potassium oxidation.
I didn't pay much attention to it until I noticed spots of yellow and orange discoloration on the corners of the cut. Any part where there was a
sharp corner was starting to get this peroxide/superoxide coating on it. Believing at the time that all peroxides were super dangerous (something
which I've later learned is a bit exaggerated. Most peroxide/superoxide accidents happened while the potassium was cut under highly flammable liquids
like kerosense or ligroin, etc), I wanted to cut off the peroxides. I saw the bulk of it on the corners of the lump, so I cut them off exposing a
fresh surface. That is when I put the lump into the container above. (And disposed of the cutoff in a nice puddle. Beautiful lilac fire resulted).
For the longest of time the fresh metal surface remained. Slowly, however, it began to take on a reddish hue. I was puzzled by this, because at the
same time the yellow spots began to shrink and little "pits" formed on the oxide crust. All the while, the red colors deepened. I had/have no clue
what that is. I do not know of ANY potassium compounds that are that color of red. (Or red at all for that matter). Noticing the little pin-hole
sized pits developing, and the disappearance of the peroxide/superoxide, I began to think that perhaps it was a normal potassium oxide formation, but
of the VERY thinnest of amounts. If the jar is air-tight, then no further oxidation from atmospheric oxygen could happen. (And based on my
sodium/lithium sample that may be the case). After seeing numerous different anondized metals, I thought that maybe a similar action was happening
here with my potassium.
\"A real fart is beefy, has a density greater than or equal to the air surrounding it, consists of the unmistakable scent of broccoli, and usually
requires wiping afterwards.\"
http://maddox.xmission.com. |
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Today I cut a piece of sodium off a larger block in the lab I work(ed) at. The only oil I could find was some parrafin oil which was opened 30 years
ago, and the bottle had not been used or moved in 10 years. I put the chunk of sodium in there, and it is the best damn oil I have ever used to store
sodium. The sodium is still as shiny as when it was first cut. Must be all the air in the oil left the oil during the extended storage time without
being disturbed.
I had never bothered to pretreat my mineral oil with sodium before, so I was amazed how much difference this makes.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Is mineral oil the best cover oil for sodium? I don't have any but see it on the shelves at the pharmacies. I have some odorless BBQ starter fluid
which I believe is essentially kerosene. This seems like it would be easier to blot off the sodium when you want to use it. Or does it make any
difference?
I don't have any sodium but plan to make it soon. 
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
It is traditional to store Na and K under rock oil, kerosine, or some similar high boiling cut; before using the freshly cut pieces would be quickly
blotted and then rinsed with toluol or xylol, and perhaps finally with petroleum ether.
Mineral oil has the advantage that it is very difficult to ignite. In some cases you don't need to do a real thorough cleaning job, it's boiling range
is above many if not most substances being synthesised and so would be left behind during distillation.
|
|
|
ordenblitz
Hazard to Others
  
Posts: 259
Registered: 18-7-2004
Location: Northwest
Member Is Offline
Mood: Bohr'd
|
|
Does anyone happen to know how much the hydroxide layer that forms on the surface of sodium, will slow down the further conversion of the metal? If
one had say a 5" diameter log of Na with a 1/8 hydroxide sealing layer. How long could one expect the piece to last? Just looking for opinions since
stumbling on a great deal for 6.6 pounds of the stuff in two big logs. I have sealed them in several PE bags and placed each in a new paint can.
I have been thinking about what to do for the long term since this should last me my lifetime if taken care of properly. Maybe getting some 1 qt paint
cans and hacking off smaller ~1 lb pieces, flushing with argon and soldering the tops on. But possibly that is overkill if these logs just develop a
good 1/4” to1/2” thick surface layer and stop there.
Hmm what to do.
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
@ordenblitz: I know that I found a 3" diameter by 2" inch 118g piece of sodium in the stockroom (making Na methoxide) that was at least 25 years old
and had never been opened. Upon opening it (in a metal can, with a screwdriver) I found that it was completely covered in its oxide and probably its
carbonate as well. The thick white layer was about 1 cm or 3/8" thick. So, for unprotected sodium that is decades old it seems it does not oxidize to
a severe extent.
I think there is a threshold where it will not progress any further into the metal. Upon cutting the rod in half, I could clearly see how thick the
layer was since the rose-colored sodium is in stark contrast with the snow-white oxide. If you want to clean yours up, you can melt the sodium under
mineral oil in a metal can or steel pipe and skim off the oxide and then 'cast' the sodium into cold oil. It will quickly solidify into shiny
irregular lumps.
Since you have pounds of sodium, would you be inclined to sell any or trade any? I know you're a fan of stockpiling reagents, but c'mon  Anyway, I'll u2u. Anyway, I'll u2u.
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
What about melting the sodium under oil and pouring it into a suitably sized wine bottle? If the bottle is filled up to the neck only a small area can
be attacked by oxygen, as opposed to the huge area present on the original lump. This seems like it would be much more suitable for long term storage.
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I also have some sodium (no, not pounds, just a few tens of grams  ), and now I
store it under colorless and clear paraffin oil. I wonder, can I store it under ligroin or petroleum ether? The fact, that it is stored under oil
keeps me off from experimenting with it. It is a real mess to get out some of the Na-metal. It would be much more convenient to store it under a
volatile liquid. When a piece is cut off, simply dab it with some cotton and then let the liquid evaporate. Nice and clean. ), and now I
store it under colorless and clear paraffin oil. I wonder, can I store it under ligroin or petroleum ether? The fact, that it is stored under oil
keeps me off from experimenting with it. It is a real mess to get out some of the Na-metal. It would be much more convenient to store it under a
volatile liquid. When a piece is cut off, simply dab it with some cotton and then let the liquid evaporate. Nice and clean.
What is the reason that nobody does this?
|
|
|
| Pages:
1
2
3 |