Keftedes89
Harmless

Posts: 12
Registered: 3-12-2014
Member Is Offline
Mood: No Mood
|
|
Will this Reaction Work?
I am trying to make good leaving groups out of the alcohol groups in dihydroxyacetone. The problem is, I don't like working with overly hazardous
materials that will kill me. I avoided the common agents that convert primary alcohols to primary haloalkanes because I don't want to deal with
dichloroacetone, dibromoacetone, or diiodoacetone.
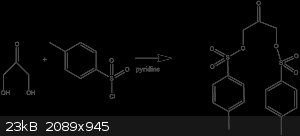
I want to know if I can use tosyl-chloride to substitute the alcohol groups for tosyl groups. Will this reaction work or will the ketone functional
group make for some trouble? Finally, is there another method I can use to convert the -OH to a safe leaving groups that wont create a compound that
will kill me?
|
|
|
forgottenpassword
Hazard to Others
  
Posts: 374
Registered: 12-12-2013
Member Is Offline
Mood: No Mood
|
|
I don't see why not. Solubility may be an issue. And pyridine isn't exactly harmless (if you're a male).
|
|
|
Nicodem
|
Thread Moved
3-12-2014 at 08:15 |
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Dunno. Got not idea what you are up to.
Now, Acetone Dicarboxylic Acid, isn't terribly difficult to decarboxylate. Does this help?
Easy enough to make. Though some opinions differ.
http://www.orgsyn.org/demo.aspx?prep=cv1p0010
[Edited on 7-12-2014 by zed]
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by zed  |
Now, Acetone Dicarboxylic Acid, isn't terribly difficult to decarboxylate. Does this help? |
I think you've made a mistake as that's citric acid being decarboxylated, and I'm not sure what that has to with the post.
You may have wanted to add in this citation: J. Phys. Chem., 1928, 32 (7), pp 961–981 DOI: 10.1021/j150289a001
As for the proposed reaction, it seems viable if you have a non-nucleophilic base to mop up the produced acid. Triethylamine, Hunig's base, etc. You
should check the pKa's of the alpha carbons and tailor your base strength appropriately.
[Edited on 8-12-2014 by Chemosynthesis]
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Chemosynthesis,
Not clear what the petitioner is trying to do. He isn't telling us exactly.
Doubtful that he is actually trying to make acetone. But, if he is, it is probably easier to do it with acetone dicarboxylic acid, than with
dihydroxyacetone.
Tell us more, Keftedes89.
[Edited on 15-12-2014 by zed]
[Edited on 15-12-2014 by zed]
|
|
|
vmelkon
National Hazard
   
Posts: 669
Registered: 25-11-2011
Location: Canada
Member Is Offline
Mood: autoerotic asphyxiation
|
|
Where is the pyridine?
Signature ==== Is this my youtube page? https://www.youtube.com/watch?v=tA5PYtul5aU
We must attach the electrodes of knowledge to the nipples of ignorance and give a few good jolts.
Yes my evolutionary friends. We are all homos here. |
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
It's under the 'reaction arrow', as the solvent. Wouldn't pyridine be able to 'mop up', as proposed?
I agree on suspicious-ness, as the poster is worried about toxicity.
|
|
|