The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Building a flow battery: control electronics
Has anyone here ever done this?
For anyone not familiar, a flow battery is pretty much a reversible fuel cell. Instead of just pumping stuff through it, you have the ability to
charge it regenerating reactants.
Now this is not simply a mental exercise here, I am looking at converting my place to solar, so the idea of a battery that lasts almost forever and
that I could build myself appeals. Depending on my electronics knowledge, hence this thread
I am specifically looking at a vanadium redox battery
http://en.wikipedia.org/wiki/Flow_battery
http://en.wikipedia.org/wiki/Vanadium_redox_battery
http://energystorage.org/energy-storage/technologies/vanadiu...
http://www.vanadium-redox-battery.com/vanadium-redox-battery...
because it seems that regardless of how good your membrane is, you are going to get exchange across so the electrolytes in those cases do not last
forever. So despite vanadium being more expensive than iron and chromium or bromine / polysulfide I want to build this type. Plus, vanadium has nice
colors.
So while I am confident on the chemistry and the physical building, the control electronics are where I am confused.
How do I create a method of charge measurement for the battery? If I overcharge, I will no longer be doing the desired electrochemical reactions, but
instead start on water electrolysis which will change pH and potentially precipitate the V if it continues. So I want some sort of method that when
the cell is fully charged, the charging source is disconnected.
Can any of the electronics gurus recommend anything?
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
One of your refs claims a 2-8 hour storage duration. In other words, not enough to really help for solar applications.
http://energystorage.org/energy-storage/technologies/vanadiu...
| Quote: | | The cell voltage is 1.4-1.6 volts and cell power densities are 100’s mW/cm2 (although Prudent Energy reports their power densities are higher). The
DC-DC efficiency of this battery has been reported in the range of 60-80%. According to EPRI, the vanadium redox battery is suitable for power systems
in the range of 100 kW to 10 MW, with storage durations in the 2-8 hour range. |
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
I am not sure how they arrive at that 2-8h range. You have two tanks, one of a V(IV) V(V) couple and the other a V(II) V(II) pair depending on charge
state. These are not going to spontaneously undergo redox behavior unless flowing through the cell with a load, so there is no reason for it to lose
charge over time. They are almost as isolated as two strips of metal before you stick them in a lemon to make a battery 
they sell commercial VRBs for solar use, so it must be possible http://www.americanvanadium.com/cellcube.php#
[Edited on 30-11-14 by The_Davster]
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Thanks The_Davster for that link.
I have sent off a request for a quote. I already have a solar system and the appeal of going totally off-grid is quite high. (Making my own is
likely to be problematic for me for more than just the reasons you have encountered.)
J.
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
I believe that "storage durations" in this context refers to how many hours the battery can operate at full power before needing to recharge. In
principle you could make a VRB with large energy capacity but moderate power to operate for days or weeks, but currently there does not seem to be a
commercial niche for such systems.
PGP Key and corresponding e-mail address
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Aaah. That makes sense. Although it is rather an unusual phrasing to use if that is what was meant.
For my domestic situation it is conceivable that I would have a daily deficit of up to 6kWh for a couple of weeks at a time over winter. (In summer
my daily excess supply from solar panels is 14-15kwH most days.) I would want sufficient storage capacity to see me through the winter patch if I was
to consider going off grid. Another kW of solar panels and a generator backup may be warranted. The costs might be prohibitive, but it is worth
looking into. Locally the electricity costs have gone up 91% in the last five years with no end in sight.
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
What has me particularly interested in this battery is that it becomes very simple to add more capacity...just use larger tanks of electrolyte! But
problem is the energy density
A Tesla model S has a 60 kWh battery.
So a VRB has a power density of around 20 Wh/L. To get the same energy content you need 3000L of electrolyte
or 1500L for each half cell, which is the size of a giant rain barrel or really big fish tank.
Assume concentration of 2M V2O5 = 6000 mol V2O5=~1000 kilos V2O5

And for shits and giggles...to power my house for a month I would need over 5000 kilos of dissolved vanadium pentoxide
[Edited on 1-12-14 by The_Davster]
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Are there any special requirements for the tanks though?
Could a couple of polymer rainwater tanks be used? If so, they are readily available around here. There are also bladders that can be fitted into
the space under the floor of the house. They need not take up much space.
If these could be used, that would seem to me to be the easy part of the project. Finding a few thousand kilograms of vanadium pentoxide at a
reasonable price might be a bit harder.
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
I've seen reference to polypropylene tanks, but considering it is just vanadium pentoxide, sulfuric acid, and maybe some HCl to bump up solubility, I
also don't think tank material is the limiting factor.
Alibaba would be the place to begin to look for this much vanadium pentoxide.
I just did a search on there...free sample of vanadium pentoxide....nifty.
http://www.alibaba.com/product-detail/Vanadium-Pentoxide-Fre...
Also, I have read papers about this stuff that claims a VRB the size of a medium fridge could be enough for a home solar system.....yet my calculation
above seems to use much, much more.
This is because I did just jack the capacity of a tesla battery for no real reason.
One solar installation I read about recommended an 18 kWh storage set up...which would significantly reduce vanadium need.
[Edited on 1-12-14 by The_Davster]
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Lots of useful info in this ppt presentation
http://www.eurosolar.org/new/pdfs_neu/electric/IRES2006_Skyl...
especially this:
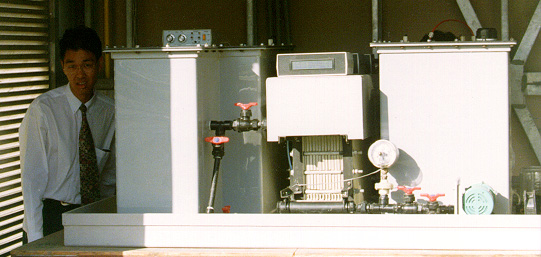
this is a prototype 1kw/12 kwh system.
[Edited on 1-12-14 by The_Davster]
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
V2O5 does a great thermite. (Don't blink though. It's fast.) See MrHomeScientist's youtube for an example.
It is also useful for making H2SO4.
I ordered myself some -- if they will send it to me.
[/aside]
That system you show is a bit bulkier than I expected for 1kW and 12 kWh. That would scale quite big if you wanted to keep a house running.
[edit] I should look more closely. It is sitting on a bench. And most of it is the two tanks which are reasonably
slim.[/edit]
I will look at the presentation later.
[Edited on 1-12-2014 by j_sum1]
|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
Quote: Originally posted by The_Davster  | Has anyone here ever done this?
For anyone not familiar, a flow battery is pretty much a reversible fuel cell. Instead of just pumping stuff through it, you have the ability to
charge it regenerating reactants.
Now this is not simply a mental exercise here, I am looking at converting my place to solar, so the idea of a battery that lasts almost forever and
that I could build myself appeals. Depending on my electronics knowledge, hence this thread
I am specifically looking at a vanadium redox battery
http://en.wikipedia.org/wiki/Flow_battery
http://en.wikipedia.org/wiki/Vanadium_redox_battery
http://energystorage.org/energy-storage/technologies/vanadiu...
http://www.vanadium-redox-battery.com/vanadium-redox-battery...
because it seems that regardless of how good your membrane is, you are going to get exchange across so the electrolytes in those cases do not last
forever. So despite vanadium being more expensive than iron and chromium or bromine / polysulfide I want to build this type. Plus, vanadium has nice
colors.
So while I am confident on the chemistry and the physical building, the control electronics are where I am confused.
How do I create a method of charge measurement for the battery? If I overcharge, I will no longer be doing the desired electrochemical reactions, but
instead start on water electrolysis which will change pH and potentially precipitate the V if it continues. So I want some sort of method that when
the cell is fully charged, the charging source is disconnected.
Can any of the electronics gurus recommend anything?
|
While I would have to find time to study this battery in order to understand fully the conditions which would be monitored in order to create a design
fulfilling your needs a few ideas jump out from reading your post. If I understand you correctly you need a way to interrupt the charging voltage when
the battery reaches certain conditions. If one knows the terminal voltage at which point the battery is fully charged one could design a comparator
circuit which simply opens a relay or SSR when the voltage rises to a defined value. You mention PH and I wonder if building a circuit around an
Arduino to meet your requirements is a better method. Say you used a voltage input as one parameter and also wrote the code to monitor a PH probe so
that when the PH was reached where your 'water electrolysis' begins this signal also could be used to open the circuit from the charging source. An
'OR' comparison, PH reaching 'bad' range or voltage reaching a certain value, either value tripping the circuit. Some batteries need the current to be
monitored, or temperature, meaning not only is an intelligent circuit a good idea, one can also alter control thresholds by changing values stored in
program code.
For a probe I can think of two items:
http://www.amazon.com/Aquarium-Hydroponic-controller-Electro...
And the probe circuit:
http://www.amazon.com/Atlas-Scientific-CIRCUIT-FOR-ARDUINO/d...
A better probe:
http://www.amazon.com/American-Marine-PINPOINT-pH-Probe/dp/B...
No doubt higher quality probes and circuits exist but it does not seem like you need to spend more for your application. Going further other sensors
could look at precipitate in the cell and activate a suction pump (cell designed for it) which pulls the bulk of sediments out. Also liquid levels
could be monitored by other sensors where refilling could be automated by other pumps or valves. Of course one would want say the 2560 Mega Arduino if
several inputs are to be monitored and several automated functions are required. Just thinking out loud as I say I have not had time to study this
type battery and I could be imagining far greater complexity than you require. If you merely need to open a charging voltage source when the battery
reaches a set voltage a simple comparator-relay circuit would work. What is the voltage when your cell is at full charge and how many charging amperes
do you expect (maximum value expected)?
"Science is the belief in the ignorance of the experts" Richard Feynman
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Thanks IrC! this gives me a great starting point for further research.
The voltage at full charge will depend on how many cells I decide on in series, something i am researching now. Similarly, in terms of charging
amperage, I need to decide on a solar system first to determine this. thanks!
jsum: I also went for the free V2O5 sample, but never heard from them. Did you get a response?
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
For the Controller, what exactly would you need ?
Current measurement and the ability to automatically reverse the polarity from time to time ?
|
|
|
froot
Hazard to Others
  
Posts: 347
Registered: 23-10-2003
Location: South Africa
Member Is Offline
Mood: refluxed
|
|
I have always wondered about connecting cells in series, which is not avoidable, and the effects it has on the electrolyte considering it has contact
with the first through to the last electrode in the series stack. This would mean there is a voltage difference between the electrode plates and what
sort of effect would this have on the electrolyte even if it is in close proximity to the electrodes for a short period of time.
Edit. I would use a cell voltage sensor to control the electrolyte pump speed.
[Edited on 6-12-2014 by froot]
We salute the improvement of the human genome by honoring those who remove themselves from it.
Of necessity, this honor is generally bestowed posthumously. - www.darwinawards.com |
|
|
chief3
Harmless

Posts: 39
Registered: 29-12-2012
Member Is Offline
Mood: No Mood
|
|
I like this idea very much, and was recently thinking about such a battery ; only : Cant there be any harmless stuff used ? If one of the tanks fails
the environmental departement will call a geologist, who will estimate you have to pay 5 000 000 $ for recovery of the soil under the house ... ...
I for example have a lot of space, and e few basins in the cellar could be made, each of 5000 liter , wouldnt miss the space ... .
There are also cheap salts , eg. Na2SO4 ... ; this could be electrolyzed through a membrane (of bricks) to H2SO4 and NaOH ... , with no
electrode-corrosion ; those 2 reactants give usually quite an exothermic reaction when unified ... so potentially also quite some kWh ... ; low
voltages at many amperes can be handled ... by alternators of copper and iron, no semiconductors ... and can be transformed to usable voltages ... eg.
with modified welding-transformers ...
The membrane would have to be made of thin bricks and 2 sorts of mortar: Standard for the alkaline side of the cell, and some acid-resistant stuff for
the acidic side ... eg. clay or something ... ...
What do you guys think about that ? ??
================
The electrode-materials would be:
==> stainless steel or even plain steel for the alkaline side ... ; historically electrolysis-cells for production of H2 were made this way, with
Na2CO3 as the electrolyte ...
==> probably carbon-rods for the acidic side ... ... , the sort used for welding ... ; or what else ? lead-sheet ?
================
By the way Na2SO4 has an interesting thermodynamic equilibrium with water, wehrer it stores lage amounts of thermic energy at 32 [Celsius] ... which
makes it interesting for thermal storage of heat-energy too ... for heating of the floor ... ...
Mabe there even could be made a cell which can store both: Thermal energy _and_ electricty ...
[Edited on 15-12-2014 by chief3]
[Edited on 15-12-2014 by chief3]
[Edited on 15-12-2014 by chief3]
|
|
|
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
I get the feeling there is a great deal more than just voltage monitoring and temperature etc.
The electronics would be simple with a micro. the hard part is knowing which parameter/parameters need monitoring. I cant see pH being a good choice,
if you think about the size of the unit and depending where you place the probe there could be lag before the probe detects the rise/fall. So do you
have an idea what actually begins to happen at the point the battery starts to over charge? What starts where? There are some batteries where you
measure temperature then have to continue to charge for X amount of time before you disconnect (I really dont have a clue which now).
Then you have the maintenance of a probe, they wonder off of calibration pretty quick sometimes. The only thing I can say is find out what actually
begins to happen when you reached max charge, then you have a start point, but to be honest just about ANY modern micro would handle this with ease.
Dont ask me, I only know enough to be dangerous
|
|
|
chief3
Harmless

Posts: 39
Registered: 29-12-2012
Member Is Offline
Mood: No Mood
|
|
Supposed one has a hypothetical [Na2SO4 <--> NaOH + H2SO4]-Cell ...
==> On overcharge it might just produce Hydrogen and Oxygen ... ...
I think this could be tired with some simple setup, maybe I'll go for it these days ... ; just wonder about an inert electrode for the H2SO4-part of
the cell ...
[Edited on 16-12-2014 by chief3]
|
|
|
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
Quote: Originally posted by chief3  | Supposed one has a hypothetical [Na2SO4 <--> NaOH + H2SO4]-Cell ...
==> On overcharge it might just produce Hydrogen and Oxygen ... ...
I think this could be tired with some simple setup, maybe I'll go for it these days ... ; just wonder about an inert electrode for the H2SO4-part of
the cell ...
[Edited on 16-12-2014 by chief3] |
Maybe then if you measure the voltage from the charge unit behind a diode and then measure the voltage of the cell at the same time, there should be a
difference that is fairly constant (at least trackable), on over charge you would detect a sudden drop or rise in voltage or current.
If it was me I would build something small scale and measure current and voltage going in and the voltage current of the cell, I would then log until
you get overcharge. Like I say measuring is easy, knowing what to measure at what will change electrically is the harder part.
Dont ask me, I only know enough to be dangerous
|
|
|
WChase501
Harmless

Posts: 16
Registered: 23-1-2014
Location: Hot Springs Arkansas
Member Is Offline
Mood: Depressed and Happy
|
|
What controls are you interested in?
"Playing God" is false, Nothing in our Universe is impossible, you just need to know the method of doing whatever you want to. Knowledge is
everything.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
A surprising observation
Supposedly applying a voltage regulator to an ordinary cell battery allows it to be deep discharged extracting up
to 7 times the normally available duty cycle, according to the claim made by the inventor.
http://www.pcworld.com/article/2928997/batteriser-is-a-250-g...
http://www.google.com/patents/US20120121943
Are batteries really that inefficient ?
.
|
|
|
Bright Spark
Harmless

Posts: 23
Registered: 1-3-2015
Member Is Offline
Mood: No Mood
|
|
How do I create a method of charge measurement for the battery? If I overcharge, I will no longer be doing the desired electrochemical reactions, but
instead start on water electrolysis which will change pH and potentially precipitate the V if it continues. So I want some sort of method that when
the cell is fully charged, the charging source is disconnected.
Davster, I work for a company that makes products that uses electrolysis stacks, I don't know very much about them as its not something I have ever
been involved with outside of curiosity
To make control electronics can seem really difficult at first but its more accessible these days then it has ever been
You need a micro controller to do all these things, its steep learning curve but with a bit of enthusiasm its certainly achievable
|
|
|
Sulaiman
International Hazard
    
Posts: 3695
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
I would not worry about the charging electronics yet,
batteries of similar chemistry but different construction do not always have similar end of charge voltage or charge capacity or allowable maximum
current.
Build a prototype cell,
measure everything whilst charging at a constant current (voltage, pH, temperature etc)
stop charging when a significant quantity of bubbles are seen.
Discharge the cell and do the same at a different charging current to check for anomalies.
If none of the measured variables give a good indication of end of charge,
then you will have to regulate the battery charge by total charge.
That would probably need to be digital as an analogue integrator would be difficult to design for a one year integration time.
I'm sure that you will get all the help needed with the electronics design,
if not I volunteer to design a single cell charger for you,
except for the charge integration system as I have promised myself not to go back into programming.
(I started in '76 with predictive electronic ignition at Lucas using 8048, 1K ROM / 64 Bytes RAM ! )
... things got more complicated after that, and I get 'sucked in' and ignore my family
I too am concerned about the electrolytes forming a conduction path between cells,
At least one break in continuity for each electrolyte stream is required,
some sort of batch transfer system, could be as simple as a drip feed.
I think that using bricks etc. as the semi-permeable membrane will give a high internal resistance,
significant energy will be lost both during charge and discharge.
I have experimented with small photovoltaics and I have three partially completed panels using 5" and 6" square cells,
D.I.Y. assembly of solar panels is extremely tedious.
Here in central UK we get equivalent to 5 hours sun per day in summer, 0.5 hours per day in winter,
the most economical solution is to sell power to the grid in summer and pay for it in winter.
Any chance of wind power to supplement winter consumption?
[Edited on 7-6-2015 by Sulaiman]
|
|
|
Texium
|
Thread Moved
22-11-2023 at 19:29 |