blindreeper
Unregistered
Posts: N/A
Registered: N/A
Member Is Offline
|
|
reduction of syphnic acid
I was doing some thinking. Picric acid (trinitrophenol) and styphnic acid (trinitoresocinol) are very similar. Picric acid is reduced to picramic acid
with sodium sulfide. This in turn is made into DDNP.
I lack the great knowledge of organic chemistry, but could styphnic acid be reduced to something similar to picramic acid but with an extra OH and
then made into a DDNP type substance (diazodinitroresorcinol)?
I think this is all crazy talk from a childish mind but you never know it could work.
Any comments?
If anyone has some syphnic acid/resorcinol and alot of time maybe they could try this.
Also if this is not new and been dicussed before please excuse me.
|
|
|
fluffy bunny
Harmless

Posts: 25
Registered: 17-10-2002
Location: Australia
Member Is Offline
Mood: No Mood
|
|
Yes that is certainly possible! Theoretically, any aromatic-amine can be diazotised.
However, there could be certain difficulties that would make it impossible to make, and i don't mean to sound negative, but if it were a useful
primary, it probably would have been documented already.
But i certainly think it would b an interesting experiment, with caution.
|
|
|
KABOOOM(pyrojustforfun)
Hazard to Others
  
Posts: 254
Registered: 12-10-2002
Location: Iran (pseudoislamic dictatorship of)
Member Is Offline
Mood: exuviating!
|
|
the next case after s<b>t</b>yphnic acid is trinitrophloroglucinol. <a
href="http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PALL&p=1&u=/netahtml/srchnum.htm&r=1&f=G&l=
50&s1=4,434,304.WKU.&OS=PN/4,434,304&RS=PN/4,434,304">Patent US4434304</a> is <i>Synthesis of
trinitrophloroglucinol</i>.
|
|
|
Madog
Hazard to Others
  
Posts: 221
Registered: 20-5-2002
Location: USA
Member Is Offline
Mood: lysergic
|
|
yeah, i bet it would be good but the sensitiveity might be bad. i had the same idea a little while ago
Most people outgrow their pyro tendencies, we are the ones who\'s tendencies outgrew us.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Styphnic acid: C6H(OH)2(NO2)3
OB: -35,9%
Volume of detonation gases: 680L/kg
density: 1,83g/ccm
lead block test: 284 ccm/10g
impact sensitivity: 7,4 Nm
heat of explosion (H2O l): 4271 kJ/kg
VOD :?
Picric acid: C6H2(OH)(NO2)3
OB: -45,4%
VODG: 800L/kg
density: 1,767g/ccm
LBT: 315 ccm/10g
IS: 7,4 Nm
heat of explosion (H2O l): 4522 kJ/kg
VOD : 7350 m/s @ 1,7g/ccm and confined
Picramic acid: H2N-C6H2(OH)(NO2)2
OB: -76,3%
VODG: 800L/kg
density: ?
LBT: 166 ccm/10g
IS: 34 Nm
Heat of explosion (H2O l): 2722 kJ/kg
VOD: ?
DDNP: C6H2(-O-)(-N=N-)(NO2)2
OB: -60,9%
VODG: ?
density: 1,63g/ccm
LBT: 326 ccm/10g
IS: 1,5 Nm
HOE : ?
VOD: 6600 m/s confined @ 1,5g/ccm
1)From the above datas, you can conclude that styphnic acid has a better density and a better OB than picric does; but since it contains more H will
produce more H2O what will reduce its heat of explosion!
Anyway in the salts of those acids there will be less H and here you may conclude that the heavy salts of those will be stronger primaries than the
ones of TNP!
TNPhloroglucidates > Styphnates > picrates in VOD, density, heat output, OB, brisance!
Sensitivity is the same for the pure acids and TNPG should also be close!
2)The transformation of TNP into PAA lowers the density and the OB but reacting PAA to DDNP yields an explosive of better LBT and similar VOD but with
lower OB and much higher sensitivity than the original TNP!
3)The formation of DDNP brother of styphnic acid and TNPG will lead to free acid -OH groups that will be able to make salts!
The free acid will already be much more sensitive than TNP and their salts must be even more sensitive!
(HO)C6H(-O-)(-N2-)(NO2)2 looks like TNP
(HO)2C6(-O-)(-N2-)(NO2)2 looks like TNR
With stronger reductors one might have:
didiazonitroresorcinol C6H(O)2(N2)2(NO2)
didiazonitrophloroglucidol HO-C6(O)2(N2)2(NO2)
tridiazophloroglucidol C6(O)3(N2)3
Which should have initiating habilities but maybe are too sensitive!!!
    
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Also something that would be much more interesting would be to nitrate picramic acid (maybe after protection of the NH2 by acylation) with conc HNO3
and H2SO4 to get 3,4,5,6 tetranitro-2 aminophenol (moderate sensitive very HE)
The later can also make a DTeNP primary of much higher energy output!
C6(O)(N2)(NO2)4

PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
KABOOOM(pyrojustforfun)
Hazard to Others
  
Posts: 254
Registered: 12-10-2002
Location: Iran (pseudoislamic dictatorship of)
Member Is Offline
Mood: exuviating!
|
|
knowing the fact that aromates having -NH2 and -OH in para position also form diazooxides, we can predict more explosive compounds.
for instance diazotation of nitration product of para-aminophenol(made by hydrolyzation of acetaminophen tablets) sounds interesting. all u have to
do is to add the tablets in a dilute acid solution, cool it down, collect the crystals, add the crystals to mixed acid, diazotiate the product to get
brother of DDNP. it is easier to make than DDNP, as there's no reduction step.
acetaminophen is very easy to purchase (cheap too)
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
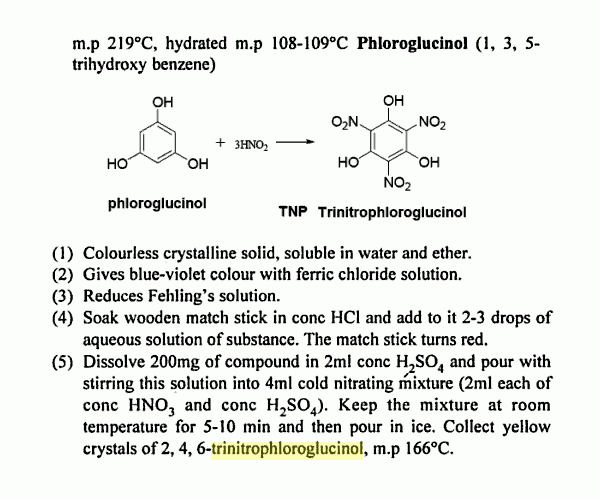
An Improved Preparation of Trinitrophloroglucinol
A. A. Defusco; A. T. Nielsen; R. L. Atkins
Organic Preparations and Procedures International: The New Journal for Organic Synthesis,
Volume 14, Issue 6, (1982) Pages 393 – 395
This is the patent posted above _
Synthesis of Trinitrophloroglucinol US4434304
Studies on 2,4,6-trinitrophloroglucinol (TNPG) A novel flash sensitizer
Mehilal, N Sikder, A K Sikder, D V Survase & J P Agrawal
Indian Journal of Engineering & Materials Sciences , Vol. 11, February 2004, pp. 59-62
2,4,6-Trinitrophloroglucinol (TNPG), useful for percussion caps, detonator
formulations and dye manufacture, has been synthesized with conventional
nitrating agents under mild reaction conditions. The compound is characterized
by IR, NMR, mass and elemental analyses. Further, the purity of the compound
is confirmed by estimation of nitro groups. Thermal and explosive properties of
TNPG have been investigated. The detonation velocity and detonation pressure
are also estimated.
http://chemicalland21.com/lifescience/phar/1,3,5-TRIHDROXY%2...
The major shortcoming to the syntheses of trinitrophloroglucinol is that
phloroglucinol is about $50 a pound in bulk quantity. Trinitrophloroglucinol
and triaminotrinitrobenzene synthesis from inexpensive nitroarenes such
as picric acid are detailed in the following patents _
Synthesis and Purification of 1,3,5-Triamino-2,4,6-Trinitrobenzene (TATB)
This is the patent _ US 7057072
Synthesis of Trinitrophloroglucinol and Triaminotrinitrobenzene (TATB)
This is the patent _ US 7057073
Related threads _
http://www.sciencemadness.org/talk/viewthread.php?tid=5406
http://www.sciencemadness.org/talk/viewthread.php?tid=4457
.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Hi Franklyn,
I'm interested...you wrote:
"Thermal and explosive properties of
TNPG have been investigated. The detonation velocity and detonation pressure
are also estimated."
Can you write those data down?
The link under only goes to 1,3,5-trihydroxybenzene and related compounds...TNPG lead salt CAS number is wel listed but not the datas  (CAS 51325-28-1) (CAS 51325-28-1)
Thank you.
PHZ
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by PHILOU Zrealone  |
I'm interested...you wrote:
"Thermal and explosive properties of TNPG have been investigated.
The detonation velocity and detonation pressure are also estimated." |
PHILOU Zrealone
I don't have the article , It's just the abstract shown here _
http://www.niscair.res.in/ScienceCommunication/ResearchJourn...
Unfortunately the free access journals don't include those from Vol 11 , 2004
http://nopr.niscair.res.in/handle/123456789/36
The only source I could find is this _ http://cat.inist.fr/?aModele=afficheN&cpsidt=15541560
A related paper is available from here _ http://dx.doi.org/10.1016/j.jhazmat.2007.01.043
these are best requested in the references section.
.
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
This was the materia (2,4,6-Trinitrophloroglucinol)l that originally put me off Legard (Preparatory Manual of explosives). Not only could I not find
ONE of his sited patents (this was the 2nd edition I think) but the synthesis was exceedingly wasteful of acid.
|
|
|
Peroxid
Hazard to Self
 
Posts: 56
Registered: 23-8-2006
Member Is Offline
Mood: No Mood
|
|
Hi!
Here is this (http://dx.doi.org/10.1016/j.jhazmat.2007.01.043 ) reference about TNPG.
Attachment: TNPG2.pdf (200kB)
This file has been downloaded 1678 times
|
|
|