| Pages:
1
2 |
Tricia
Harmless

Posts: 23
Registered: 27-10-2014
Member Is Offline
Mood: No Mood
|
|
Homework Questions-
So I've been given an NMR for a compound that has carbon, hydrogen, and oxygen in it. It has a molecular weight of 114 g/mol. My NMR is showing a
triplet at the 1.0 ppm range with 3 hydrogens detected from it, a multiplet around 1.5 ppm detecting 2.02 hydrogens, and another triplet around 2.5
ppm detecting 1.98 hydrogens. Based upon the NMR alone, it looks like 7 hydrogens are attached to carbons.
There is 73.6% carbon and 12.3% hydrogen. I took the difference of this and got 61.3% oxygen. When calculating the empirical formula I got 6.1 mols
for carbon, 12.3 mols for hydrogen, and 3.8 mols for oxygen, and so when I calculate the formula I got this: C1.6H3.2O1.
Now this is where I start running into difficulties with my calculations. When I added up the number of carbons, hydrogens, and oxygens in the
empirical formula I came back with 38.4 grams. When I divide that from 114 g/mol I got 2.96 mols. I thought I needed to multiply this back into the
empirical formula but the numbers ended up wrong. I keep getting a molecular formula that doesn't look right to me. My most recent attempt yielded
this formula: C5H9O3.
Can someone help me with what I'm doing wrong? Thanks!
[Edited on 31-10-2014 by Bert]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4334
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Here's your problem. What should these percentages add up to?
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Tricia
Harmless

Posts: 23
Registered: 27-10-2014
Member Is Offline
Mood: No Mood
|
|
Oh my God, you're absolutely right! I just realized having the 61.3% would put it WAY over 100! No wonder I wasn't coming back with the right answer!
Thank you!
Okay, after adding 73.6 and 12.3 together I got 85.9. When I subtracted that from 100 I got 14.1 - which should hopefully be the correct concentration
for oxygen! Thanks again!
|
|
|
Tricia
Harmless

Posts: 23
Registered: 27-10-2014
Member Is Offline
Mood: No Mood
|
|
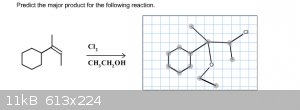
Predict the Major Product for the Following Reaction
I'm out of tries with this one homework question so I just want to make sure I have the correct reaction drawn before I submit this answer. I tried to
follow Markovnikov's rule and attach the Cl to the sp2 carbon with the most hydrogens and I attached the CH3CH2O- to the one with the least hydrogens.
Am I pretty close? Thanks!
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
You are correct. The alcohol is nucleophilic towards the chloronium intermediate and generates an ether favoring the more substituted area. Just make
sure they are in the anti position.
The mechanism is given at the bottom of page 3 here.
|
|
|
Tricia
Harmless

Posts: 23
Registered: 27-10-2014
Member Is Offline
Mood: No Mood
|
|
Thank you!
|
|
|
Tricia
Harmless

Posts: 23
Registered: 27-10-2014
Member Is Offline
Mood: No Mood
|
|
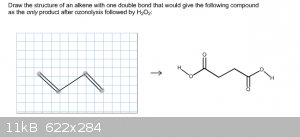
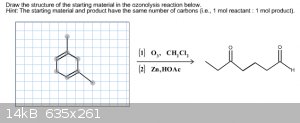
More Homework help
I'm starting to realize that I'm not very good at ozonolysis. I think I've got the hang of it with my online homework, but with the following two
questions I'm literally on my last try with these. I've drawn out in both what I believe to be the correct starting formula. Would anyone be able to
tell me if I'm correct? If I'm not, could you please point out to me what I'm doing wrong?
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
this is brilliant ,i thought that the alcohol was only a solvent in the reaction ,so the normal halogenation should be done only in non nucleophilic solvent to get dihalogenated products.but can haloamines also be formed this way
or will the bromine just attack the amine to form a quaternary ammonium salt?
,so the normal halogenation should be done only in non nucleophilic solvent to get dihalogenated products.but can haloamines also be formed this way
or will the bromine just attack the amine to form a quaternary ammonium salt?
also ,if you study the reaction mechanism ,in the normal dihalogenation a carbocation is formed whereas the cohalogenation proceeds via a bridge
intermediate(no carbocation formed) so there is no chance of a rearrangement even if a more substituted carbon is available .
but if only dihalogenation is done ,is there any chance of a ring expansion
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
ok ,so your first compound is a dicarboxylic acid (butane dioic acid) whereas your second compound is an aldehyde derivative with an oxo group(the
aldehyde is given more priority to the keto while naming the compound)
now you must first understand why its different although the same ozone has been used
that's because in the first conversion ,oxidative ozonolysis is used (that's why there is H2O2 ) which will oxidise the
intermediate ozonide to acid
the second conversion uses reductive ozoloysis(that's why Zn/acetic acid has been used .the Zn supplies electrons to the ozonide intermediate and the
solvent donates the protons(H+ as it is an acid) to protonate the ozonide,resulting in the formation of the aldehyde and the keto groups
and Zn takes away one of the O of the ozonide to form Zinc oxide(ZnO)
now the best way to get back the starting compound which has been ozonolysed is to just join the two carbons with the double bond O with a double bond
(first rub away the two O and then just join the two carbon atoms with a double bond)
so for example if your ozonolysis product was 2 molecules of formaldehyde(H2C=O) then by following the above method you can easily tell
that that the starting product was ethene((H2C=CH2)
now back to your first compound,by following the method you see that by joining the first and the last carbon atoms with a double bond after removing
the two O(ignore the -OH ,they are there because of the peroxide) ,you get a ring compound(which should look like a square or rectangle with a double
between the two carbon atoms you just joined ) this is called cyclobutene
doing the same thing with the second compound(join the rightmost carbon and the 5th carbon with a double bond you get another ring compound ,but a
part of you final product i.e the two carbons(-C2H5 group)just to the left of the 5th carbon is outside the ring, forming 1-
ethyl cyclopentene (the numbering is important as you have to specify the location of the ethyl group(Et) )
[Edited on 30-10-2014 by CuReUS]
[Edited on 30-10-2014 by CuReUS]
|
|
|
Tricia
Harmless

Posts: 23
Registered: 27-10-2014
Member Is Offline
Mood: No Mood
|
|
Now I get it! Thank you!
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
Just for future reference, you may consider making a homework thread in Beginnings, since that section is devoted more towards homework (even in
o-chem, as seen in the textbook thread in that subforum: https://www.sciencemadness.org/whisper/viewthread.php?tid=66...).
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Yes- Homework questions should be in "beginnings", and a single thread seems best for now...
I'll help you out by collecting all these homework threads...
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Bert
|
Threads Merged
30-10-2014 at 17:03 |
Bert
|
Threads Merged
30-10-2014 at 17:06 |
Bert
|
Thread Moved
30-10-2014 at 17:07 |
Tricia
Harmless

Posts: 23
Registered: 27-10-2014
Member Is Offline
Mood: No Mood
|
|
Stereochemical structures
Okay, this one has me really confused. The question asks me to draw the structures that are part of the fumarase-catalyzed reaction. I got the product
correct but I keep getting the reactant incorrect. The structure drawn in the picture below that is marked with a red X is the incorrect answer. I thought this was the structure for fumarate, but it was incorrect. I also thought this was trans
but it still coming up wrong. Do I have the overall structure correct but have the placement of oxygens wrong? Or am I completely off the mark?
Thanks!
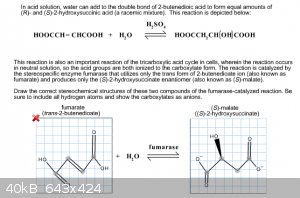
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
The structure is correct but the software you're using doesn't appear to like your wonky bond angles on the left hand side.
|
|
|
Tricia
Harmless

Posts: 23
Registered: 27-10-2014
Member Is Offline
Mood: No Mood
|
|
You're correct. Once I redrew the structure and straightened out that one double-bonded oxygen, then it said my answer was correct. Thank you!
|
|
|
Tricia
Harmless

Posts: 23
Registered: 27-10-2014
Member Is Offline
Mood: No Mood
|
|
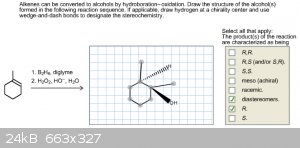
Stereochemistry problems
Some of my online homework problems are really puzzling me. After a previous question of mine posted basically led to the revelation that my structure
was technically correct, but for some reason didn't look correct in the program, I'm wondering if I'm yielding the same issue with other problems. The
two structures below are ones I thought I had correct but I honestly can't tell if I'm completely wrong or if I'm drawing the stereochemistry
incorrectly. The first one in the list I've drawn the Br attaching to the alkene with different wedges and dashes attached at different points and
it's still incorrect. With the second one I know that the BH2 that's supposed to attach to one of the double bonds forms an OH. Is this just a matter
of me not following syn/anti addition correctly with these two problems?
|
|
|
Dr.Bob
International Hazard
    
Posts: 2734
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
In the left example, I think there are more products possible, so try to figure out what they might be.
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
I think the issue in the left example is that when you draw a chiral tetrahedral carbon, two of the bonds can be in-plane (normal lines), one has to
be dashed and one has to be a wedge.You have 3 bonds in-plane for both carbons, although your bromines are correct. Dr.Bob also has a point in that
there is another possible product.
For the right example, you seem to have added an extra methyl onto the carbon with the OH. BH3 addition to a double bond is a syn process, and the
oxidative cleavage to the alcohol preserves the stereochemistry. You've drawn the product of anti-addition. Additionally, there are two possible
products since the borane can attack the double bond from either side.
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
Bert
|
Threads Merged
5-11-2014 at 13:12 |
Bert
|
Threads Merged
5-11-2014 at 13:16 |
Tricia
Harmless

Posts: 23
Registered: 27-10-2014
Member Is Offline
Mood: No Mood
|
|
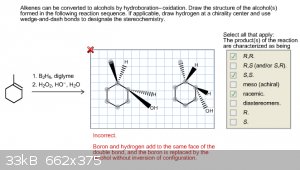
Draw the structure of the alcohol(s) formed in the following reaction sequence.
I posted this in the incorrect thread so it got deleted before I could see if anyone had responded to it. I've been stumped about this one for awhile
though, and right now I want to check and make sure I have the setup for these correct but simply have the placement of the atoms wrong. Below is my
most recent attempt at this problem and by the hint I think I'm on the right track, but I'm on my last try with this problem so I want to make sure I
get this right. So, am I really close and just have some placements wrong? Or am I completely off? Thanks!
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Your regioselectivity is correct. But, you've got the stereochemistry wrong. Boron and hydrogen add to the same face, and the oxidation proceeds with
retention of configuration at boron, so you end up with H and OH on the same face; this is not what you've drawn.
Whether you need to draw both diastereomers when you've ticked the "racemic" box on the right hand side is to be determined.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
tricia ,please post all your questions in this thread
http://www.sciencemadness.org/talk/viewthread.php?tid=41534#...
i think your question has been moved to the above thread,not deleted
[Edited on 6-11-2014 by CuReUS]
|
|
|
Bert
|
Threads Merged
6-11-2014 at 08:13 |
Tricia
Harmless

Posts: 23
Registered: 27-10-2014
Member Is Offline
Mood: No Mood
|
|
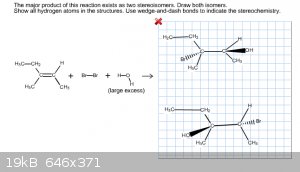
Finding Two Stereoisomers
This is the tricky thing with online chemistry homework in that I don't know if a structure I draw is wrong because of stereochemistry, or if it's
wrong because the whole thing is wrong. So I've got this problem below, and I thought with the excess H2O that there would be an OH- to attach to the
structure. Does this mean that there is an HBr leftover on the side for bother stereoisomers? I forgot to draw the HBr's in the problem, but am I at
least on the right path?
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
You have the right idea; this is producing a vicinal haloalçohol and the addition is anti... however, you mistakenly drew only one Markovnikov
product. You will get a racemic mixture of Markovnikov stereoisomers as your main product.
You will produce HBr, but it's generally of little significance.
[Edited on 7-11-2014 by Chemosynthesis]
|
|
|
Tricia
Harmless

Posts: 23
Registered: 27-10-2014
Member Is Offline
Mood: No Mood
|
|
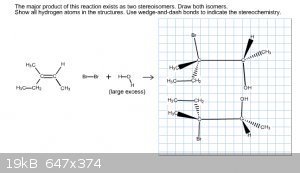
I'm ready to pull my hair out over all these problems. It would be better if examples of these excess problems were in my textbook, but they're not! I
can't even find anything on the internet relating to this! So after solving the trans portion (thank you for your help Chemosynthesis) I thought it
would be a cakewalk to solve the cis version of the same problem. But I keep getting it wrong. And again, I don't know if it's because my
stereochemistry is incorrect or because my structures are completely wrong. I have the image below of my recent try with this. Do I have the
stereochemistry wrong or is this wrong because I drew it wrong?
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
Your stereo chemistry would be correct, but you placed the bromine and hydroxyl group incorrectly, so your regiochemistry is mistaken.
When performing a Markovnikov addition, the most electronegative group adds to the most substituted or stable carbon. Oxygen is more electronegative
than bromine, so alcohols or ethers form on the most substituted carbon here.
Some confusion may come from acid addition; If it were HBr addition, bromine is more electronegative than hydrogen, so the bromine would be on the
more substituted carbon. If you have elemental bromine in an inert, aprotic solvent, the dihalide is equally electronegative and so it doesn't matter.
Edit: if you don't want to just memorize this, think about the mechanism that is occurring. In organic chemistry, you will see a pattern of
nucleophiles attacking electrophiles. The way we draw electron arrows is supposed to convey this, because your negative charge is what you start the
arrow from.
When you look at your products here, the double bond is a source of electron density, and so if there is a reaction, it will attack the least
electronegative group. In the case of HBr, this is hydrogen. In your example, it is bromine. Now you are stuck with a positive charge in your
resonance structures. Consider where it is most stable. Your remaining nucleophile (excess water with the lone pairs on oxygen, solvating your
reactant) logically attacks this and adds to that carbon.
[Edited on 7-11-2014 by Chemosynthesis]
|
|
|
| Pages:
1
2 |