| Pages:
1
2 |
pdb
Hazard to Self
 
Posts: 90
Registered: 8-4-2004
Member Is Offline
Mood: No Mood
|
|
Cyanuric Triazide
It is always a nice day when the opportunity is given to prepare a new primary... This one is reported to be dual, both primary and HE (VoD about 5500
m/s and high power due to absence of heavy metal cation). It also has a melting point of 94°C without decomposition, which may open new or specific
uses.
Synthesis
- 2.50 g NaN3 were dissolved in 10 ml water in a 50 ml beaker
- 15 ml acetone was added, and the solution warmed up to 45°C with stirring
- 1.55 g C3N3Cl3 (sand-like easy flowing dense fine white powder, with an unpleasant smell-dry fish !) were added
temperature and strong magnetic stirring were maintained for 30 minutes; if stirring is off, the solution separates into two layers: the upper layer,
which is the smaller one, seems to be an acetonic phase of dissolved C3N3Cl3. It is thus necessary to entertain strong stirring so as to create an
emulsion which will maximize contact surface between the two phases
- acetonic phase decreases little by little, and is gone after 20-25 minutes. Simultaneously, white flakes of C3N12 precipitate
- beaker is left at room temperature for five more minutes, and then poured at once in 150 ml of ice water under strong magnetic stirring
it’s difficult to state if extra precipitation occurs, as the initial flakes are already there
- after 5 minutes, the solution is decanted and filtrated, and the precipitate washed with cold water (C3N12 is insoluble). The white flakes appear to
be crystalline aggregates
- when dry, C3N12 is made of white crystals, same granulation size than table salt
- yield is 83% of theory, i.e. 1.42 g. Given the fact NaN3 must be in excess amount, this is certainly one of the best synthesis to waste precious
sodium azide ! (conversly, the Rosco’s azo-clathrates probably make the best possible use of it).
(attached is a picture of the main steps)
CTA detonates at flame contact with no noticeable DDT. Mechanical sensitivity not tested yet. Dropped in boiling water, the crystals melt immediately
and gather in globules whose size and number depend on the intensity and velocity of stirring. By progressive addition of cold water or slow cooling,
these globules solidify, yielding to a dense free-flowing powder. CTA specific density is 1.5 g/cc.
[Edited on 2-7-2005 by pdb]
[Edited on 2-6-2008 by vulture]
Picture removed - Too large - please resize to 800x600 and reupload
[Edited on 2-6-2008 by vulture]
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Very interesting, pdb.
Why is it called 'cyanur' triazide, isn't the starting compound C3N3Cl3 1,3,5 trichloro 2,4,6 triazine, whose monomer is a very nasty
carcinogenic and toxic compound, cyanogen chloride(NC-Cl))?
(Whereas cyanur chloride, or trichloro isocyanuric acid is (O=C-NCl)3, So C3N3O3Cl3, where the chlorine is bound to the N, however. )Have I got
something mixed up with the nomenclature?
Anyway, did you make the C3N3Cl3 yourself? How?
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
The cyanuric chloride precursor is isomeric with TCCA , but is a different compound entirely . And TCCA cannot be substituted in the synthesis of
cyanuric azide .
There is an alternate route by diazotization of cyanuric trihydrazide described in the original patent .
GB170359 Manufacture of a New Explosive
I believe the cyanuric trihydrazide is likely an even more difficult precursor than is the cyanuric chloride for the better known method with sodium
azide . Cyanuric trihydrazide differs from hydrazine cyanurate which is a very easily made hydrazine-cyanuric acid salt . It might
be worthwhile to experiment with a diazotization of the easily made hydrazine cyanurate anyway , just to see if anything of interest may result .
Either of the patents methods are going to require precursors which are difficult ,
specialty organic compounds . And given the reported storage stability issues for
cyanuric triazide , along with its expense of production due to the difficult precursors , it seems to be more of an interest as an academic curiosity
, than a practical material . A method using more common precursors would increase interest in the material . But its stability issues would still
rule out its acceptance for any commercial use because of more stable alternatives which have become
well proven standards .
|
|
|
pdb
Hazard to Self
 
Posts: 90
Registered: 8-4-2004
Member Is Offline
Mood: No Mood
|
|
I used commercial grade C3N3Cl3.
Rosco: I think quite hard to find extensive information about CTA beyond common knowledge (Fedoroff, Urbanski, COPAE, GB patent etc).
However, I own minutes from some evaluation tests. They report spontaneous detonations when growing large crystals (quite common with primaries), and
also when drying amorpheous powder obtained by sudden precipitation in ice water (less common). But they do not say anything about stability of molten
CTA once solidified. Any information on this precise feature would be welcome.
They also describe ignition tests of CTA/TNT mixures, 80:20 and 50:50. The former detonates at flame contact.
I agree CTA is more an lab curiosity than a compound to consider for industrial uses. However, my interest in this stuff is only driven by its ability
to be loaded in molten state.
[Edited on 2-7-2005 by pdb]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Davis reports that it melts at 94 C but
decomposes above 100 C . And it is also reported that there are sometimes explosions when the finely crystalline material is press loaded . The
material
is slightly hygroscopic and also slightly volatile , requiring the use of sealed caps .
None of these properties are in favor of the material being used in comparison to
better alternatives .
In the next article in COPAE , Davis describes trinitrotriazidobenzene made by reaction of sodium azide with trichlorotrinitrobenzene , another
difficult precursor . An interesting experiment might be to nitrate paradichlorobenzene moth crystals , using a sulfuric and nitrate mixture , to
form trinitrodichlorobenzene ,
and then do a similar reaction with sodium azide to form trinitrodiazidobenzene , which may also have properties of a primary explosive .
It should have good stability and power .
I have never seen the compound mentioned anywhere . So this is one for pure experimentation .
|
|
|
Axt
National Hazard
   
Posts: 818
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
<center>I like numbers.</center>
<center><table border="1" cellpadding="5" bgcolor="FFFBE8" ><tr><td
rowspan="2"><font size="2"><center><b>AZIDE</td><td
colspan="3"><center><b>Pendulum Friction</td><td rowspan="2"><center><b>Impact
fall<br>500g weight</td><td
rowspan="2"><center><b>Explosion<br>temperature</td></tr><tr><td><font
size="2"><center><b>Added weight in kg</td><td><font
size="2"><center><b>Fall</td><td><font size="2"><center><b># of
swings</td></tr><tr><td><font size="2"><center>Pb(N<sub>3</sub> <sub>2</sub></td><td><font
size="2"><center>0.45</td><td><font size="2"><center>37.5</td><td><font
size="2"><center>12</td><td><font size="2"><center>43</td><td><font
size="2"><center>383°C</td></tr><tr><td><font
size="2"><center>AgN<sub>3</sub></td><td><font
size="2"><center>4.35</td><td><font size="2"><center>33.0</td><td><font
size="2"><center>30</td><td><font size="2"><center>41</td><td><font
size="2"><center>273°C</td></tr><tr><td><font
size="2"><center>HgN<sub>3</sub></td><td><font
size="2"><center>1.00</td><td><font size="2"><center>50.0</td><td><font
size="2"><center>16</td><td><font size="2"><center>6</td><td><font
size="2"><center>298°C</td></tr><tr><td><font
size="2"><center>(CN)<sub>3</sub>(N<sub>3</sub> <sub>2</sub></td><td><font
size="2"><center>0.45</td><td><font size="2"><center>37.5</td><td><font
size="2"><center>12</td><td><font size="2"><center>43</td><td><font
size="2"><center>383°C</td></tr><tr><td><font
size="2"><center>AgN<sub>3</sub></td><td><font
size="2"><center>4.35</td><td><font size="2"><center>33.0</td><td><font
size="2"><center>30</td><td><font size="2"><center>41</td><td><font
size="2"><center>273°C</td></tr><tr><td><font
size="2"><center>HgN<sub>3</sub></td><td><font
size="2"><center>1.00</td><td><font size="2"><center>50.0</td><td><font
size="2"><center>16</td><td><font size="2"><center>6</td><td><font
size="2"><center>298°C</td></tr><tr><td><font
size="2"><center>(CN)<sub>3</sub>(N<sub>3</sub> <sub>3</sub></td><td><font size="2"><center>0.00</td><td><font
size="2"><center>12.5</td><td><font size="2"><center>3</td><td><font
size="2"><center>7</td><td><font size="2"><center>252°C</td></tr></table> <sub>3</sub></td><td><font size="2"><center>0.00</td><td><font
size="2"><center>12.5</td><td><font size="2"><center>3</td><td><font
size="2"><center>7</td><td><font size="2"><center>252°C</td></tr></table>
<table border="1" cellpadding="5" bgcolor="FFFBE8"><tr><td
rowspan="2"><center><b>Charge<br>Weight</td><td colspan="4"><center><b>Weight of sand
crushed finer then 30 mesh</td></tr><tr><td><font size="2"><center><b>Mercury
fulminate</td><td><font size="2"><center><b>Lead azide</td><td><font
size="2"><center><b>Silver azide</td><td><font size="2"><center><b>Cyanuric
triazide</td></tr><tr><td><font size="2"><center><i>grams</td><td><font
size="2"><center><i>grams</td><td><font
size="2"><center><i>grams</td><td><font
size="2"><center><i>grams</td><td><font
size="2"><center><i>grams</td></tr><tr><td><font
size="2"><center>0.10</td><td><font size="2"><center>-</td><td><font
size="2"><center>-</td><td><font size="2"><center>3.3</td><td><font
size="2"><center>4.8</td></tr><tr><td><font
size="2"><center>0.20</td><td><font size="2"><center>3.8</td><td><font
size="2"><center>5.9</td><td><font size="2"><center>6.8</td><td><font
size="2"><center>12.2</td></tr><tr><td><font
size="2"><center>0.30</td><td><font size="2"><center>8.0</td><td><font
size="2"><center>-</td><td><font size="2"><center>10.4</td><td><font
size="2"><center>-</td></tr><tr><td><font
size="2"><center>0.40</td><td><font size="2"><center>12.2</td><td><font
size="2"><center>12.7</td><td><font size="2"><center>-</td><td><font
size="2"><center>33.2</td></tr><tr><td><font
size="2"><center>0.50</td><td><font size="2"><center>16.0</td><td><font
size="2"><center>16.1</td><td><font size="2"><center>18.9</td><td><font
size="2"><center>-</td></tr><tr><td><font
size="2"><center>0.60</td><td><font size="2"><center>20.1</td><td><font
size="2"><center>20.9</td><td><font size="2"><center>-</td><td><font
size="2"><center>54.4</td></tr><tr><td><font
size="2"><center>0.75</td><td><font size="2"><center>-</td><td><font
size="2"><center>-</td><td><font size="2"><center>30.0</td><td><font
size="2"><center>-</td></tr><tr><td><font
size="2"><center>0.80</td><td><font size="2"><center>28.2</td><td><font
size="2"><center>28.5</td><td><font size="2"><center>-</td><td><font
size="2"><center>68.9</td></tr><tr><td><font
size="2"><center>1.00</td><td><font size="2"><center>36.8</td><td><font
size="2"><center>33.6</td><td><font size="2"><center>41.1</td><td><font
size="2"><center>78.6</td></tr></table></center>
<center><i> Taylor and Rinkenback: J. Franklin Inst. 204, 369 (1927).</i></center>
[Edited on 2-7-2005 by Axt]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Numbers are good if you factor in everything , like accounting for the
percentage of the metallic component
as if it were " ballast " , and then
looking at the impulse developed
when that " ballast " is hurled by
the energetic component , to arrive
at how efficiently is the energy released ,
on an azo group by azo group basis .
And then there's also the matter of density , which has bearing on the potential output per unit volume of charge , more important really in
measuring practical explosive " power "
in terms of ability to do actual work ,
than sand test figures for given weights
of charge which have great density differences .
These kind of factors that apply greatly
when device " A " is acting upon
target " B " and which blows the biggest hole in such tests , using identical size
devices , is highly relevant for detonators .
|
|
|
Axt
National Hazard
   
Posts: 818
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
True the numbers dont usually lie, but the interpretation of them might.
More figures of interest.
<i>"According to Kast and Haid (141) it develops a maximum detonation velocity of approximately 7500 meters per second when compressed to a
density of 1.54, whereas the figure for lead azide with density of 4.6 is 5300 meters per second." Kast & Haid: Z. angew. Chem. 38, 43
(1925).</i>
EDIT: Relationships for compression to VOD's.
Cyanuric triazide
1.4g/cm<sup>3</sup> under 200 atmospheres per cm<sup>3</sup>
1.5g/cm<sup>3</sup> under 800 atmospheres per cm<sup>3</sup>
VOD = 5550m/s @ 1.15g/cm<sup>3</sup>
Pb azide requires 800 atmospheres to achieve 3.5g/cm<sup>3</sup> (crystal density is 4.79g/cm<sup>3</sup> . VOD = 4500 @ 3.80g/cm<sup>3</sup>. . VOD = 4500 @ 3.80g/cm<sup>3</sup>.
ref. Muraour: Bull. soc. chim. [4] 61, 1152 (1932).
[Edited on 3-7-2005 by Axt]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
I would think that in the overall evaluation
for performance in that group above ,
silver azide is probably the shining star .
And getting above that performance requires going to tetrazoles and their
complexes .
Cyanuric triazide is powerful yes , but personally I would compare it with silver fulminate and on stability it would probably lose there , which
doesn't speak well of it .
A mixture of nitromannite and DDNP or some other primary would likely be more powerful and stable , and much less expense and trouble to make . There
are
eutectic melts possible there too which would be more stable than cyanuric triazide .
|
|
|
Lambda
National Hazard
   
Posts: 566
Registered: 15-4-2005
Location: Netherlands
Member Is Offline
Mood: Euforic Online
|
|
Sand crush test result comparison.
In the sand crush test, it looks like Mercury fulminate shows quit a long traject of DDT (deflagration to detonation transition) compared to Lead
azide. At about and above 0.3 grams they look comparatively pared in the results. Cyanuric triazide shows relatively fast DDT even as low as 0.1 gram
samples. Lead azide and silver azide behave relatively instantaneously, having a very short DDT, but less power than Cyanuric triazide. This lower
power is atributed to the high precentage of heavy metalls lead and silver.
[Edited on 3-7-2005 by Lambda]
|
|
|
pdb
Hazard to Self
 
Posts: 90
Registered: 8-4-2004
Member Is Offline
Mood: No Mood
|
|
Lamda: CTA's DDT occurs in much smaller amounts than 0.1 g. I see virtually no difference -as far as I can observe without specific measuring
device- with other primaries like Pb(N3)2, AgN3, AgONC, DPNA etc.
Rosco: a DDNP-MHN eutectic would certainly be less an hazard to handle, but stability over time might yet be questionnable because of MHN's
"fragility". But the idea deserves a try.
I don't know if your comparison between CTA and AgONC is relevant (as many, I had numerous unexpected explosions with AgONC, all due to
mechanical stresses, but no spontaneous detonation), but don't you think than CTA cristallized from melting should be less sensitive and less
exposed to sublimation ?
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
I'm interested in the precursor cyanuric trichloride C3N3Cl3.
It is NOT the isomer of TCCA (C3N3O3Cl3).
Did you buy it from a chemical supplier (if yes, which?) or somewhere else (where and as what?) ? How expensive was it?
How would one go about synthesizing it?
Maybe Cyanuric acid + thionyl chloride?
Maybe it could be prepared fron TCCA by reduction. No idea what reducing agent to use however...
Or is the trimerization of cyanogen chloride the only possibility?
Cyanogen chloride isn't that difficult to make if you're able to make NaCN or KCN.
However, there are the usual hazards in handling, given the fact that ClCN is a nasty chemical warfare agent that causes irritation and pulmonary
edema if inhaled.
[Edited on 3-7-2005 by garage chemist]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
| Quote: | Originally posted by garage chemist
I'm interested in the precursor cyanuric trichloride C3N3Cl3.
It is NOT the isomer of TCCA (C3N3O3Cl3). |
Pardon my brain fart , you are correct that cyanuric chloride is not isomeric . I honestly am not sure where I got that idea , perhaps a
misidentification in an old text , or just my own ignoring the Oxygens on TCCA . The structures are similar and related is what I should have said .
And I never actually tried the substitution of TCCA so it wouldn't hurt anything to experiment with it . I don't think it would work , but
it might .
| Quote: |
Did you buy it from a chemical supplier (if yes, which?) or somewhere else (where and as what?) ? How expensive was it?
How would one go about synthesizing it?
Maybe Cyanuric acid + thionyl chloride? |
IIRC , and that's a big " if "  , chlorination of cyanic acid
under pressure is the industrial method , and PCl5 with cyanuric acid is one lab method . , chlorination of cyanic acid
under pressure is the industrial method , and PCl5 with cyanuric acid is one lab method .
| Quote: |
Maybe it could be prepared fron TCCA by reduction. No idea what reducing agent to use however... |
Yeah , what is going to love the oxygens ,
but leave the chlorines alone .....
maybe ascorbic acid or ferrous sulfate ?
| Quote: |
Or is the trimerization of cyanogen chloride the only possibility?
Cyanogen chloride isn't that difficult to make if you're able to make NaCN or KCN.
However, there are the usual hazards in handling, given the fact that ClCN is a nasty chemical warfare agent that causes irritation and pulmonary
edema if inhaled.
|
Maybe nitrogen trichloride in dilute form in a solvent with cyanuric acid is another idea .
|
|
|
Axt
National Hazard
   
Posts: 818
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
There was an article posted into this forum regarding the preparation of cyanuric trichloride via HCN posted into this forum, though I'm damned if i
can find it now. It's posibly in references section but I'm not sure. Does anyone know the one I speak of?
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
I posted procedures to prepare TCT (cyanuric chloride, trichloro-s-triazine) by two methods:
1. From cyanogen chloride trimerization. Very hazardous procedure!
2. By chlorination of methyl thiocyanate. The product of the chlorination is a mixture from which TCT precipitates. The TCT is filtered off and
purified. The filtrate is obnoxious and toxic, containing trichloromethyl sulfenyl chloride, thiophosgene, etc. Exhaustive chlorination of the mixture
ends with carbon tetrachloride.
The second route is much safer than the first, but still needs doing in a hood.
Methyl thiocyanate can be purchased or prepared, its preparation starts with carbon disulfide. This has also been covered in my previous posts, q.v.
I am very curoious as to pdb's "commercial grade" of cyanuric chloride. What brand, what packaging, and where did you get it?
I have Merck and Acros reagent grades. Commercial grades such as made by Italy's Lonza, usually come only in 20 Kg fiber drums.
It is best to be very specific because there has been a great deal of confusion on this forum between CC (TCT) and TCCA which is a common swimming
pool chlorinator. Related but not interchangeable.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Axt
National Hazard
   
Posts: 818
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
Thanks Sauron, yep that must have been it.
http://www.sciencemadness.org/talk/viewthread.php?tid=8330&a...
So just maybe, heat Na nitrite with Na acetate giving Na cyanide and Na bicarbonate. Pour some H2SO4 into a measuring cylinder then fill with DCM. Add
the cyanide/bicarbonate until H2SO4 is neutralised (does HCN act on bicarbonate?). Decant and add 1% EtOH. Then bubble Cl2, and evaporate to dryness.
Well thats at least what I'd try, but I'm unlikely to be trying anything thats why I'm saying it and not doing it  I've just tried to simplify it as much as possible without considering yields. No
point doing 5 times the work for two times the yield. But does anyone see a problem with is? I've just tried to simplify it as much as possible without considering yields. No
point doing 5 times the work for two times the yield. But does anyone see a problem with is?
I have Merck cyanuric trichloride as well, but buying it is seldom the point, I have never done anything with it though.
[Edited on 31-5-2008 by Axt]
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Be aware that CC (TCT) is said to have a limited shelf life. Best used fresh.
I have maybe 5 Kg never opened and may discover it will all have to be replaced.
Sic gorgeamus a los subjectatus nunc.
|
|
|
pdb
Hazard to Self
 
Posts: 90
Registered: 8-4-2004
Member Is Offline
Mood: No Mood
|
|
Sauron, by "commercial grade", I just meant it was commercially available (i.e. not home-made). Should have said "reagent grade" to avoid confusion. I
don't have it right here, but I think it was 250g from Acros.
I didn't go far in my trials of cast detonators, as the many quotes of spontaneous explosion in the literarure have somewhat chilled my enthusiasm...
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Patent for
Tris (5-amino tetrazolo) triazine
US - 20080169051
http://www.pat2pdf.org/patents/pat20080169051.pdf
Patent for
Tris tetrazolyl trazine
WO - 2008060366
http://v3.espacenet.com/captcha?original_requestUrl=http%3A%...
Synthesis of Carbon Nitride ( C3N4 )
http://handle.dtic.mil/100.2/ADA359222
Analog of Silicon Nitride theoretically deemed harder than diamond
this what happens when there are only C - N bonds
Evaluation of Cyanuric Triazide compound detonator
http://www.dtic.mil/ndia/2006fuze/mehta.pdf
How much is really known about the structure of Cyanuric triazide ?
From vol 6 of Comprehensive Heterocyclic Chemistry III
Posted by kmno4 here _
http://www.sciencemadness.org/talk/viewthread.php?tid=7208&a...
seen here below is an observation I found in subpart 6.07 Tetrazoles page 299
can this be analogous to the isomerism of Benzotrifuroxan I spoke of here
http://www.sciencemadness.org/talk/viewthread.php?tid=2969&a...
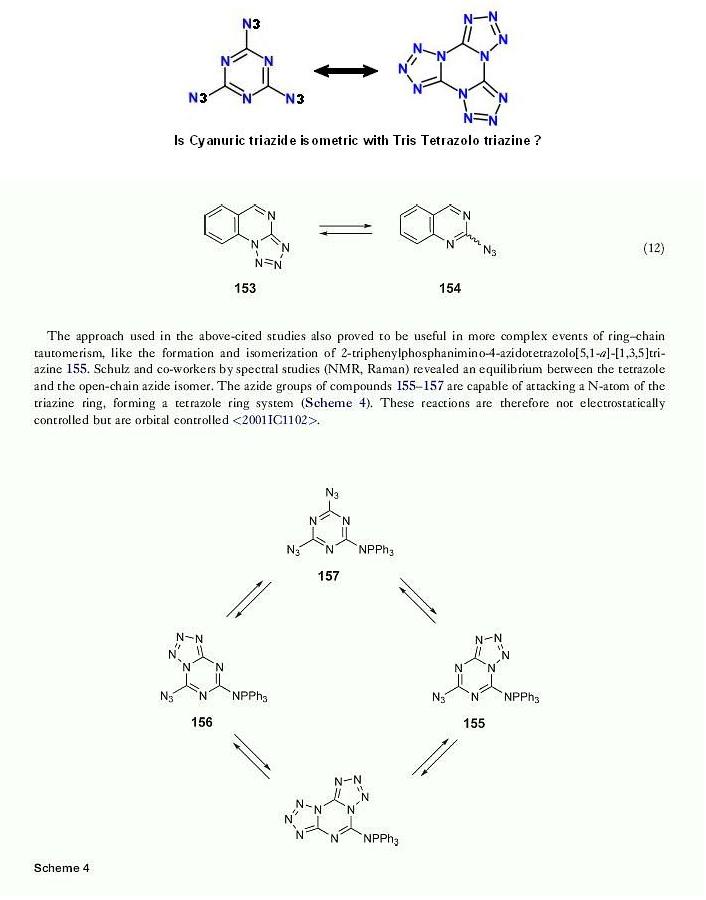
|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
| Quote: | Originally posted by Sauron
I posted procedures to prepare TCT (cyanuric chloride, trichloro-s-triazine) by two methods:
1. From cyanogen chloride trimerization. Very hazardous procedure!
2. By chlorination of methyl thiocyanate. The product of the chlorination is a mixture from which TCT precipitates. The TCT is filtered off and
purified. The filtrate is obnoxious and toxic, containing trichloromethyl sulfenyl chloride, thiophosgene, etc. Exhaustive chlorination of the mixture
ends with carbon tetrachloride.
The second route is much safer than the first, but still needs doing in a hood.
Methyl thiocyanate can be purchased or prepared, its preparation starts with carbon disulfide. This has also been covered in my previous posts, q.v.
I am very curoious as to pdb's "commercial grade" of cyanuric chloride. What brand, what packaging, and where did you get it?
I have Merck and Acros reagent grades. Commercial grades such as made by Italy's Lonza, usually come only in 20 Kg fiber drums.
It is best to be very specific because there has been a great deal of confusion on this forum between CC (TCT) and TCCA which is a common swimming
pool chlorinator. Related but not interchangeable. |
I've also found information that cyanuric chloride can be prepared from potassium thiocyanate and chlorine, watch picture below. However i was unable
to locate synth procedure for this method, may be you can find it?

|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
This should be no surprise since cyanuric chloride os also produced by chlorinating methyl thiocyanate, and again SCl2 is a byproduct. KSCN is a lot
cheaper than MeSCN.
Therefore the main problem will be figuring out how to isolate and purify the cyanuric chloride (better known around this forum as CC or TCT and not
to be confused with TCCA.
Beilstein will be the place to look for the Liebig citation. I would bet the paper appeared in Justus Liebig's Annalen der Chemie, but maybe not.
Papers that old are sometimes skimpy on experimental details, and anyway you can count on it being in archaic chemical German. Do you reaqd German?
Sic gorgeamus a los subjectatus nunc.
|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
No, but i understand some words. I can use software translator with text obtained by recognition in finereader programm.
[Edited on 20-3-2009 by Engager]
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
I can translate german articles if you ask me via U2U.
Especially if it's something as interesting as synthesis of TCT without HCN (not that HCN would be a problem for me, in fact, I would have to buy some
thiocyanate if I wanted to do the KSCN method, whereas I have plenty of ferrocyanide to generate HCN with).
But time and especially motivation is the limiting factor for me.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Take up garage chemist's offer, I have experience with machine translation of OCR and it varies from bad to awful depending on the image quality of
the pdf from which the ocr was done.
A German chemist doing the translation cannot be improved upon.
Apart from looking in the Haupywerk (Beilstein) you can look in PATR 2700, and the three volume "s-Triazine and Derivatives" and these have been made
available on the forum before.
I am sure that gc knows already but engager, I hope you have a fume hood because chlorination of KSCN will form ClCN in situ and that is what
trimerizes to the TCT - just as in the prep from MeSCN
So there is certainly the potential for release of cyanogen chloride, which is a military class chemical weapon (although an obsolete one) possesing
the blood-agent effects of HCN combined with the insidious pulmonary effects of phosgene - delayed edema without immediate warning irritation. Be
aware and take appropriate precautions.
The only method of preparing TCT that does not present this hazard is chlorination of cyanuric acid but the reagent for this is PCl5 and I am unaware
of any other. Thionyl chloride does not work. I think SbCl5 would work but do not know.
[Edited on 21-3-2009 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
The ref is actually Liebig in Pogg. Ann. 34, pg. 604-5, which can be found here. The procedure is also in the 7.Auf. of Gmelin under KSCN, who mistake KSCN for KCN. The reason the method never gained popularity likely is
because 4-5% of the used KSCN yields the cyanuric chloride. Machine translations suck, I can translate it a bit later.
[Edited on 20-3-2009 by Formatik]
|
|
|
| Pages:
1
2 |