| Pages:
1
2 |
Anarchist
Harmless

Posts: 19
Registered: 18-12-2002
Location: nowhere and everywhere
Member Is Offline
Mood: No Mood
|
|
Nitric Acid
Where do you guys get nitric acid? I know it can't be bought in any OTC products. I was thinking of making it myself from KNO3 + H2SO4 but I don't
have any kind of tubing that wouldn't get eaten away by the NO2 fumes. Is the only good place to get it from a lab?
|
|
|
trinitrotoluene
Hazard to Others
  
Posts: 142
Registered: 17-10-2002
Location: California
Member Is Offline
Mood: paranoid
|
|
This is what I suggest get 2 flask and put rubber stopers on them one have 2 holes 1 for the tubing and 1 for the thermoneter. And the other stopper
with only 1 hole. To make the stoppers resistant to HNO3 just wrap teflon on it. Do a good job on that and on both of the flasks. Then take aluminium
or glass tubing and connect the two flask togeather. And the flask your planning to use as a condenser put it into a beaker into a ice bath.I guess
this is as much as i want to explain.
TNT
|
|
|
Anarchist
Harmless

Posts: 19
Registered: 18-12-2002
Location: nowhere and everywhere
Member Is Offline
Mood: No Mood
|
|
thanks, I'll try it.
|
|
|
lucifer
Harmless

Posts: 21
Registered: 23-11-2002
Member Is Offline
Mood: No Mood
|
|
I get my hno3 from a drugstore.
I always say I use it for etching
Copper. (like printed circuit boards).
Farmers use hno3 also for disaffection.
Maybe that will help you further.
|
|
|
Anarchist
Harmless

Posts: 19
Registered: 18-12-2002
Location: nowhere and everywhere
Member Is Offline
Mood: No Mood
|
|
I don't think my drugstore carries HNO3 unless they have it behind the counter where they keep the prescription stuff. I think you're supposed to use
HCl for copper etching also. I will check it out though.
|
|
|
trinitrotoluene
Hazard to Others
  
Posts: 142
Registered: 17-10-2002
Location: California
Member Is Offline
Mood: paranoid
|
|
I know the nearest drug store I went to last time I ask them for citric acid they said they dont have it but they can order it and they told me to
wait a week. I can't freaking find citric acid i've went to many food stores and srug stores but I hope they will get me my citric acid soon. After
that I will ask them can they order some nitric acid.I hope it wont be too expensive because it seems like chemicals from the drug store is pretty
expensive like 112 grams of KNO3 it cost me $2.75
TNT
|
|
|
DeusExMachina
Hazard to Others
  
Posts: 136
Registered: 14-10-2002
Location: pakistan
Member Is Offline
Mood: No Mood
|
|
hey lucifer, what did you buy the HNO3 from the drug store as? I am desperately in need of some.
|
|
|
trinitrotoluene
Hazard to Others
  
Posts: 142
Registered: 17-10-2002
Location: California
Member Is Offline
Mood: paranoid
|
|
Its much cheaper to make a nitric acid still and make your nitric acid. You will get a higher concentration too. Thats what I'm about to do. If you
can do that then most stuff you can nitrate with H2SO4/KNO3, NH4NO3 or NaNO3. Also a cheap method. All I need is some High purity Al foil,a 2000 ml
beaker a smaller beaker like 150 ml ,and some suport and i have a HNO3 still like brainfevers but more efficiect. I arieady have 454 grams of KNO3 8
lbs of NaNO3 and 300 grams on HN4NO3. I also have 1 liter of 93% H2SO4 but i can buy gallons at the local hardware store for $12.98 its the cheap kind
thats dyed black.
TNT
|
|
|
DeusExMachina
Hazard to Others
  
Posts: 136
Registered: 14-10-2002
Location: pakistan
Member Is Offline
Mood: No Mood
|
|
thanks but I don't have any chems right now so I'd rather buy it than try to make it right now. PLus, I will have to by glass tubing and the way that
madscientist told me how to do it over AIM, I would have to put holes in my stoppers 
|
|
|
lucifer
Harmless

Posts: 21
Registered: 23-11-2002
Member Is Offline
Mood: No Mood
|
|
DeusExMachina : I bought a blue can of 25 L 58% HNO3,
This is in holland, so maybe not that useful for you.
It also wasn’t that expensive about 2,5 Euro the liter.
This was about a year ago.
|
|
|
trinitrotoluene
Hazard to Others
  
Posts: 142
Registered: 17-10-2002
Location: California
Member Is Offline
Mood: paranoid
|
|
My Nitric acid and Nitric acid still.
My nitric acid still: A 500ml erlin flask I add 300 grams of powdered Sodium Nitrate NaNO3 and mixed with 195ml of concentrated sulfuric acid (H2SO4).
Then for rubber stoppers I make my own out of Al foil.And I brought a 3' Al tube at the local ace hardware its a large tube and it cost $1.99 per
feet.So connect it to the flask anf homade stoppers out of Al foil and seal it with teflon tape, so the HNO3 vapor wont escape.On the other side I did
the same Al made stoppers and teflon taping, and the flask is in a ice bath. Ok heat source is a hot plate. I also put soaked napkins on the Al tubing
so the HNO3 will condense. So about 95% of the HNO3 condense in the Al tubing and the rest condense in the condensing and cooling flask. Today when I
distilled HNO3 my yeald was 160ml of yellow HON3.I am pretty happy with my result. Oh BTW after you are done pour the foaming soloution out of the
falsk quickly it will solidifly and will be a pain to get out of the flask. So pour that byproduct out when its in liquid form. My next goal is mass
production using 900grams of powdered NaNO3 and 580ml of concentrated H2SO4. ANd I'm going to be using a 1000-1500ml flask. Hope my yeald will be
500ml! After that I plan on using kilograms and start producing liters of HNO3.
TNT
|
|
|
a123x
Hazard to Self
 
Posts: 87
Registered: 12-1-2003
Member Is Offline
Mood: No Mood
|
|
non-dstillation method
Uh, just one question I have. How can Al tubing be used like that to distill nitric acid? Shouldn't the Al react with the acid to form aluminum
nitrate and H2? Also if you look in the general chemistry section there's a thread on the production of nitric acid using calcium nitrate, strontium
ad barium nitrate also work its just that it is easiest to make calcium nitrate. Calcium nirate and sulfuric acid make nitric acid and calcium sulfate
which is insoluble. Rather than distill it you have to filter the calcium sulfate off of the nitric acid which should be doabl with a slightly
modified fiberglass cloth(its not woven really tightly so you have to slide the threads over to make it nice and tight so the fine sulfate particals
can't get through). With that method 98% H2SO4 could yield over 98% nitric acid.
|
|
|
Darkfire
Hazard to Others
  
Posts: 292
Registered: 3-1-2003
Location: California
Member Is Offline
Mood: Wondering
|
|
The best way i have seen to distill HNO3 is by brainfever;
Use: 1 100ml beaker
1 150ml beaker
hot water bath
plastc that will with stand hot hno3
salt ice bath
h2so4 and Xno3
First: Take the 100ml beaker and place it on a stand in the middle of the 1000ml beaker.
Now fill the big flask with with the correct amount of the acid and nitrate, making sure not to go above the top of the stand.
Now place the plastic in the shape of a funnel so the tip is directly above the small beaker. Tie this on securly and add the salt ice bath on the
plastic.
Place in the hot water bath and heat.
| Code: |
|\ ice / |
| \ / |
| | big beaker
|_|sb|_| |
Sb= small beaker
\"I love being alive and will be the best man I possibly can. I will take love wherever I find it and offer it to everyone who will take it. I
will seek knowledge from those wiser and teach those who wish to learn from me.\" Duane Allman
|
|
|
BASF
Hazard to Others
  
Posts: 282
Registered: 5-11-2002
Member Is Offline
Mood: hydrophilic
|
|
Yep.
As i mentioned earlier, this is a widespread low-budget-method for purifying THC.
I was very surprised such a simple setup existed, and worked well.
(replaces expensive soxhlet extractors and professional distillation setups)
A cannabis-related homepage i´ve recently found, talks about 2-3 hours distillation time for organic solvents like ethanol and petrol ether.
|
|
|
DeusExMachina
Hazard to Others
  
Posts: 136
Registered: 14-10-2002
Location: pakistan
Member Is Offline
Mood: No Mood
|
|
hey darkfire, what type of rubber can stand hot HNO3? wouldn't the acid just eat right through it? anyways, this is how I'm gonna make my HNO3.
Please tell me if this will work...  
PS- Don't make fun of me for my stupidness please 
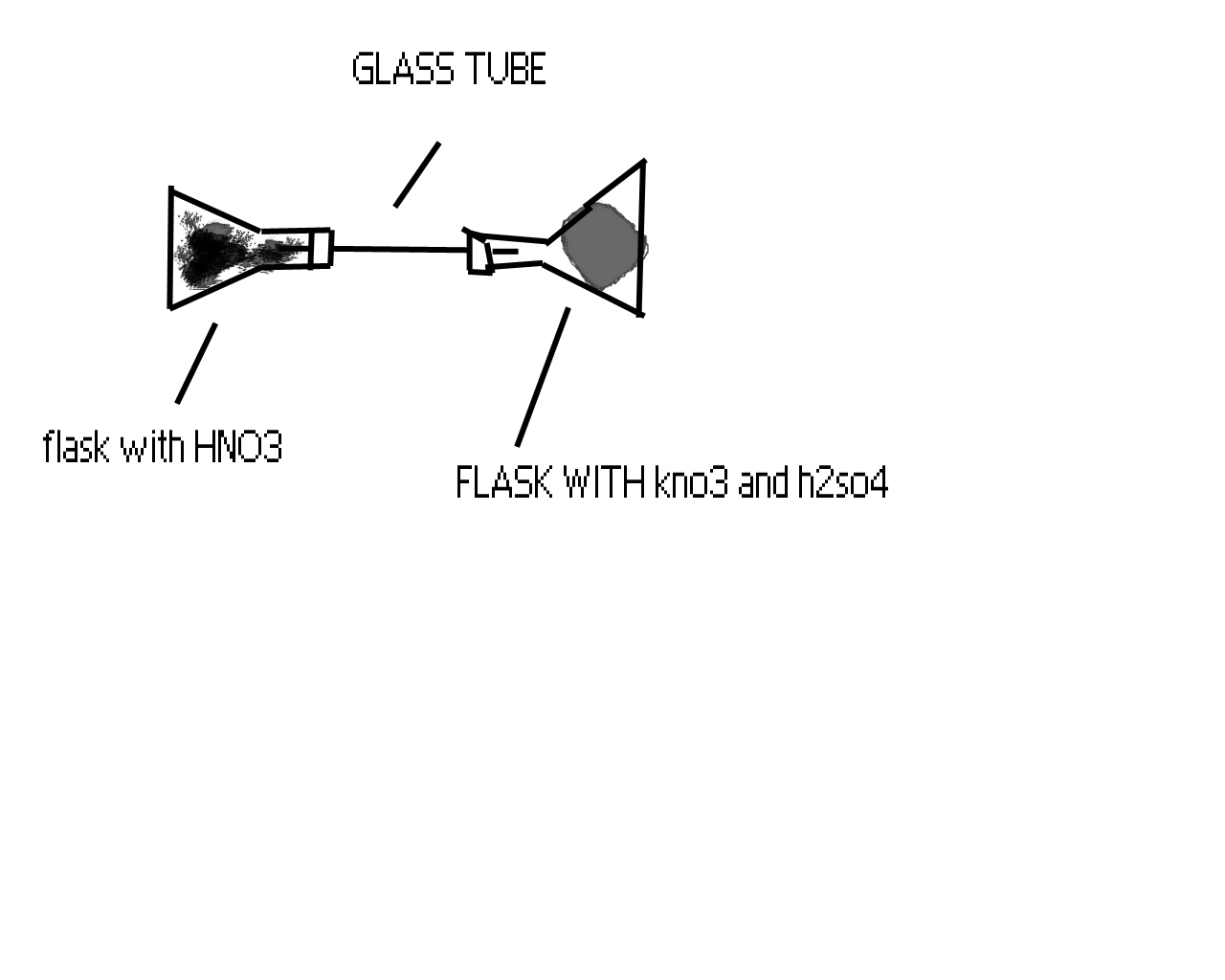
|
|
|
DeusExMachina
Hazard to Others
  
Posts: 136
Registered: 14-10-2002
Location: pakistan
Member Is Offline
Mood: No Mood
|
|
sorry for the double post (really, this edit button is pissing me off) but I forgot to include that I didn't find a rounded glass tube so this is why
the flasks have to be on there side instead of standing up.
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
Rubber won't stand nitric acid for long. I give you 5-15 minutes before it will be destroyed.
Remember that your distilling setup has to cope with hot and almost pure HNO3 gas, which is ofcourse very reactive.
Pure Al will withstand conc HNO3 because of passivation, so will iron.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
DeusExMachina
Hazard to Others
  
Posts: 136
Registered: 14-10-2002
Location: pakistan
Member Is Offline
Mood: No Mood
|
|
vulture, will my setup work? I have covered each rubber stopper with teflon.
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
Well, if you've sufficiently protected your stoppers it should work. I would advise you to cool the receiving flask because otherwise pressure build
up might cause your setup to shoot out the glass rod or the flask.
Oh, and put the receiving flask on a lower stand than the distilling flask, this will improve condensation and flow.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
DeusExMachina
Hazard to Others
  
Posts: 136
Registered: 14-10-2002
Location: pakistan
Member Is Offline
Mood: No Mood
|
|
yup, that's what I'm doing. the recieving flask is bomex glass though. Can't it crackif it gets got and then I cool it/
|
|
|
Darkfire
Hazard to Others
  
Posts: 292
Registered: 3-1-2003
Location: California
Member Is Offline
Mood: Wondering
|
|
Teflon will stand up to hno3 mabey PVC and i sure some others will, test peices before the experiment. Use Al as a backup then at least if the plastic
does melt you can save your acid from the ice bath above long enought to fix the problem. H2so4 will however eat the al foil if temp get high enought
to vapoize it.
A rubber stopper is a rubber stoper, its rubber so it wont hold up, so cover with a teflon sheet and then cover the hole where the glass tube comes in
as well.
CTR
\"I love being alive and will be the best man I possibly can. I will take love wherever I find it and offer it to everyone who will take it. I
will seek knowledge from those wiser and teach those who wish to learn from me.\" Duane Allman
|
|
|
NERV
Hazard to Others
  
Posts: 152
Registered: 22-9-2002
Location: USA
Member Is Offline
Mood: Fluorinated
|
|
From my own experience HNO3 will react with PVC, which will cause major contamination of your acid. I would stick to Teflon, and Al tubes, although
you could use PE (I think thats what it was) tubes, as from what I have herd they will stand up to HNO3 for a wile.
|
|
|
lucifer
Harmless

Posts: 21
Registered: 23-11-2002
Member Is Offline
Mood: No Mood
|
|
Coating aluminum tubes with Teflon would be ideal I guess,
But is it possible to this at home?
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Al is good to make distillation setup, as long as the process doesn't use H2SO4!
HNO3 oxydises the Al into Al2O3 what forms a protective layer!
You should then use Mg(NO3)2 or Ca(NO3)2 dehydratant to distill the HNO3!
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
rikkitikkitavi
Hazard to Others
  
Posts: 192
Registered: 17-6-2002
Member Is Offline
Mood: No Mood
|
|
most efficient dehydrant nitrate is Al(NO3)3!
Check patent GB 975324,1001615 and 1146338
for more info about using various nitrates as dehydrants.
(Al,K,Na,Ru,Li,Ca, e tc )
/rickard
|
|
|
| Pages:
1
2 |