trionic
Harmless

Posts: 9
Registered: 14-6-2013
Member Is Offline
Mood: No Mood
|
|
Please help to verify sane synthesis
Hi!
I I'm about to post a certain synth guide(Avail. on google search) for synthesis of a cyclocondensated compound.
IF it 's against rules and I'm out of line please tell me.(kindly)
I just want to know that the following is not a complete rip-off and is possible (I have solid lab experience and have looked through the theory, but
please ntoe any errors)
1. In a flask , 25 g(0.9 mol) 2-amino-2'-chloro-5-nitrobenzophenone was added to 700 mL dichloromethane and 19,45 g (0.0965 mol) Chloroacetyl
Chloride. With a stream of dry air bubbling through the mixture, the beaker is heated to 40°C for 2 h. Thereafter, the solvent is distilled off under
vacuo leaving about 35,5 g of almost pure 2-(chloroacetamido)-2'-chloro-5-nitrobenzophenone indermediate.
2. In a flask with attached water cooled condenser, 35,5g (0.9 mol) intermediate, 25.25g hexamine and 9mL 99 % formic acid is dissolved in 1250mL
absolute ethanol, the mixture is refluxed with stirring for 4 h. The ethanol is then distilled off at reduced pressure.
3.The residue from step 2 is dissolved in 500mL DCM and washed with 1000mL water, the organic soln. is then mixed with 500mL ethanol. Over a 20 cm
vigreux column the DCM is fractionally distilled off at 40°C and the residual ethanolic solution is kept cooled at 0° C for 24h where the
yellow-white crystals crash out in the cold ethanol.
Vacuum-filter the crystals on buchner and wash them with 0°C ethanol. After drying, the result is 22.5 g (80%) of a Certain Diazepine. mp.
235-237°C.
PLEASE point out any obvious errors or plain stupidity.
THank you
|
|
|
forgottenpassword
Hazard to Others
  
Posts: 374
Registered: 12-12-2013
Member Is Offline
Mood: No Mood
|
|
You are able to source these chemicals? If your 'certain diazepine' had a name it would be easier to look up a published method of its synthesis and
compare your experimental procedure with that. Nitrazepam?
[Edited on 16-6-2014 by forgottenpassword]
|
|
|
bismuthate
National Hazard
   
Posts: 803
Registered: 28-9-2013
Location: the island of stability
Member Is Offline
Mood: self reacting
|
|
Who needs sanity? Look where we are . .
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Here's the rest of his posts, with a bit more info about what he wants to do: https://www.sciencemadness.org/whisper/viewthread.php?tid=24...
|
|
|
Fantasma4500
International Hazard
    
Posts: 1681
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
yes... a small error however
''1. In a flask , 25 g(0.9 mol)''
'' , ''
|
|
|
trionic
Harmless

Posts: 9
Registered: 14-6-2013
Member Is Offline
Mood: No Mood
|
|
The complete, unmodified synthesis is in the link, Jump to step 2. I already have 2-amino-2'-chloro-5-nitrobenzophenone. So in my own modified
version, I start from Step 2 that is my Step, 1 see...
https://www.erowid.org/archive/rhodium/chemistry/clonazepam.... Clonazepam is the intended result.) And please.. THe laws in my country will
give you a fine as long as you keep the doses under 20.000 hits (2mg) it's Sched. IV
Ok, guys, the ratios and molecular weights will all be redone and double checked before any work is done.
And yes!!, I have in possesion ALL of these chems because I live in a place where its easy if you have the cash.  The precursors are ALL LEGAL too. The precursors are ALL LEGAL too.
I have all the glassware etc required for synth, but for solvent evaporation I'm thinking of using an water aspirator and patience. But maybe I should
buy a vacuum pump for like 200$? But I'm worried it will die because of solvent vapor. Though I could use a catch trap with dessicant or something.
btw. Do I need moisture protection tubes at some time during syntheszing?
STEP 2Bromoacetyl Bromide takes place of the amino group
Well, I'm going to use Chloroacetyl Chloride , that shouldnt't cause any problems right? It is used in many diazepine NH2->Clhorination rxn.
As for solvent I will substitute benzene in step 2 with DCM(methylene chloride) Just because that should dissolve nicely, right? and also evap FAST.
Most important: I just want to know that the synthesis isn't FLAWED and possibleto carry out in a decent lab setting(I have
practitcal experience and a fumehood
See below for a drawing of my slightly modified synthesis:
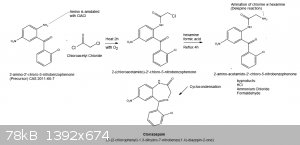
[Edited on 21-6-2014 by trionic]
[Edited on 21-6-2014 by trionic]
|
|
|
Nicodem
|
Thread Split
5-7-2014 at 04:50 |