quantumchromodynamics
Hazard to Self
 
Posts: 67
Registered: 25-9-2013
Location: with much determination, nowhere in particluar
Member Is Offline
Mood: tired but still trying
|
|
TRIETHANOLAMINE
I think I might finally be understanding something. This is an amine because it is like ammonia. Three of the hydrogen's are replaced with methanol
(ligands?). The fourth hydrogen is just gone so we have left over electrons at that position. Makes it wobbly. I realize this is pretty basic for most
of you, but this is hard for me. Is this visualization correct?
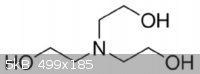
[Edited on 3-5-2014 by quantumchromodynamics]
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Um... don't you mean ethanol?
Another name for you to think about : tris(2-hydroxyethyl) amine
[Edited on 3-5-2014 by Cheddite Cheese]
|
|
|
quantumchromodynamics
Hazard to Self
 
Posts: 67
Registered: 25-9-2013
Location: with much determination, nowhere in particluar
Member Is Offline
Mood: tired but still trying
|
|
yes ethanol
Rrrr, methyl, then ethyl, i remember that...
|
|
|
quantumchromodynamics
Hazard to Self
 
Posts: 67
Registered: 25-9-2013
Location: with much determination, nowhere in particluar
Member Is Offline
Mood: tired but still trying
|
|
tris(2-hydroxyethyl) amine
I see tris(...) amine, and inside I see hydroxy, OH, but I don't get 2-hydroxyethly, why is that? How does position 2, the 2-, come into it? I thought
the numbers with the dashes at the front gave clock wise positions counting from the top for aromatic rings?
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
2: the hydroxy group is on the second carbon atom from the nitrogen
ethyl: two carbon atoms
The numbers do also give positions on rings (however, not necessarily clockwise).
|
|
|