DrChemistRabbit
Harmless

Posts: 19
Registered: 17-12-2013
Location: China
Member Is Offline
Mood: No Mood
|
|
A problem on Chlorination of Salicylic acid
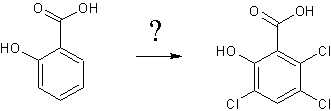
I want to synthesis this compound,but I don't know how to chloridize the Salicylic acid like the structure above.So I need some help.
Chem is try.
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
this, this and this might help.
[Edited on 2-3-2014 by DubaiAmateurRocketry]
|
|
|
bfesser
|
Thread Moved
2-3-2014 at 05:31 |
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
You could also do it by reaction with chlorine and a lewis acid catalyst (FeCl3, for example). Due to the groups attached, you will
probably get the desired substitution pattern
|
|
|